- Home
- Laboratory and Analytical Services (Services)
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
Laboratory and Analytical Services (Services)
Laboratory and Analytical Services (Services) Companies (146)
Laboratory and Analytical Services (Services) News
-
News CPHI North America a ‘one stop shop’ for professional and business development
CPHI events are renowned for connecting pharma players from across the supply chain to learn, grow, and conduct business all over the world.25 Apr 2022 -
News Lonza Switzerland site to undergo expansion of microbial development capabilities
The expansion includes the installation of a pilot suite with a 50-L fermenter and automation upgrades to accelerate clinical and commercial projects17 Nov 2021 -
News SGS completes expansion of its Biosafety Centre of Excellence in Glasgow
Clients to receive accelerated quality testing and reduced turnaround times1 Nov 2021 -
News Recipharm expands its analytical services offering in India
The CDMO inaugurates its new analytical laboratory under Recipharm Analytical Solutions25 Aug 2021 -
News Skyepharma selects Veratrak platform to ensure secure data storing and sharing
Using Veratrak's platform, the CDMO will have full oversight of data, including an audit trail of data and document processes20 Jul 2021 -
News LGM Pharma launches analytical and stability testing as standalone service
The integrated CDMO rolls out services to the broader community of drug manufacturers and developers12 Jul 2021 -
News China's Raybow Pharmaceuticals invests $15.8 million to expand US facility
The company will triple its capacity and workforce at its North Carolina site to increase its global capabilities and reach23 Mar 2021 -
News Vectura expands capabilities to develop highly potent inhaled drugs
The expansion will enable the CDMO to widen the nature of inhaled drug development projects it can undertake for customers14 Dec 2020 -
News Skyepharma Analytical Services : Specialized teams
Skyepharma is a full CDMO. We have an analytical service that covers all aspects from development to manufacturing. Packaging, raw materials and finished products are all controlled by this service.17 Sep 2020 -
News Sai Life Sciences opens new Research & Technology Centre in Hyderabad
One of India's fastest growing CDMOs goes "beyond the norm" to reinvent itself as a new generation global CDMO.17 Aug 2020 -
News EuTech announces new management team additions
EuTech has recently added two strong professionals in its Management Team. Shekhar Yerramilli has joined as COO and Rick Yglesias has joined as Head of Business Development.12 Feb 2020 -
News CDMO WuXi Biologics says drug supply unaffected by coronavirus
Amid heightened global concern over the recent coronavirus outbreak, Chinese CDMO WuXi Biologics has sought to reassure customers and patients that its drug supply lines have been unaffected.30 Jan 2020 -
News Eli Lilly unveils shared innovation laboratory in San Francisco
Lilly Gateway Labs will provide biotech companies direct access to Lilly scientists and expertise.3 Dec 2019 -
News Thermo Fisher Scientific opens Customer Solution Center in China
The new center will provide expertise in critical analytical processes and specialized workflows.20 Nov 2019 -
News Method Development, Verification & Validation of NDMA & other impurities
Accuprec Research Labs Pvt. Ltdhas successfully doneMethod Development, Verification & Validation of NDMA impurities in Ranitidine through LCMS/MS [API & Finished Dosage Form] as per USFDA method.6 Nov 2019 -
News The Leader in Spray Drying
Hovione continues to lead in Spray Drying, thanks to the largest capacity, the most experienced team of scientists that keep coming up with innovative solutions and the best-scale up science.29 Oct 2019 -
News OneVision: Creating a Global Standardized Laboratory Network for SGS Life Sciences
SGS launches OneVision, a global digitalization initiative to standardize record-keeping procedures across all Life Sciences’ testing laboratories.
27 Aug 2019 -
News How real-world data can revolutionise drug development
Utilising the current data environment and its data pools for the benefit of advancing research.9 Aug 2019 -
News MedPharm expands relationship with Novan on formulation science
New agreement allows MedPharm to react quickly to changes in requirements and makes full use of its comprehensive services for de-risking Novan’s product development.23 Jul 2019
Laboratory and Analytical Services (Services) Products (254)
-
Product Drug Substance Services
CARBOGEN AMCIS provides comprehensive Drug Substance services, offering expertise in process development, scale-up, and manufacturing of active pharmaceutical ingredients (APIs). With state-of-the-art facilities, the company specializes in high-potency compounds, including cytotoxics, and delivers solu...
-
Product Quality Management
If you trust in BioChem, you are in the best of hands, because you work together with a quality-tested full service provider. Our high-quality standards are also regularly confirmed by the authorities during inspections.
BioChem is your quality approved full-service provider: ...
-
Product R&D Services
Our R&D Services are categorized into 8 streams: • Material Science Services
• Metal and Organic Scavenging Screening Services
• Organic Synthesis Services
• Catalysis Services
• Process Services
• Chromatography and Purification Services
• Method D...
-
Product Foreign Particles
Merieux NutriSciences has developed various strategies and complementary approaches for the identification of foreign visible and subvisible particles, thanks to sophisticated instrumentation combined with a pool of experts in different fields.Download our brochure: https://bit.ly/48HKnVs
-
Product Formulation Development
Recipharm offer formulation development services for all dosage forms. We develop everything from simple formulations for early studies to more complex formulations suited for commercialisation.
-
Product Ginkgo Biloba Extract
Changsha Huir Biological-Tech Co., Ltd. is a Pre-listed professional herbal extracts and APIs manufacturer with Five Plants in China, Three Warehouses in USA(CA,NY,FL), One warehouse in Europe Changsha Huir Biological-Tech Co., Ltd. Hunan Huirui Pharmaceuticals Co; Ltd Address: Fl 20, Jin...
-
Product Analytical Services
With 55+ years of experience, our expert teams develop 1,000+ analytical methods and validate 250+ methods annually. Drawing upon an extensive range of analytical technology, combined with a wealth of analytical knowledge, we can add real value to your drug development and commercialisation programs....
-
Product Intertek Pharmaceutical Services
Intertek's pharmaceutical contract laboratory services, regulatory assistance, and supply chain assurance deliver quality and safety to meet your unique pharma and biopharma outsourcing needs. We bring quality and safety to life!
With our pharmaceutical experts working with you at every ...
-
Product Solid state characterization services
cGMP services include:- Particle size by laser diffraction on powders, sprays/aerosols, liquid dispersions - Particle size, shape & Chemical identification in powder mixtures, creams, liquids by MDRS- BET surface area and porosity- Thermal analysis DSC
Other services include:- Density (...
-
Product Lipids APIs and Intermediates
KD Pharma is the largest producer of omega-3 based APIs. Its unique technology mix produces Omega-3 drug substances of the highest quality and purity. We offer concentrates with EPA up to 99% purity, DHA up to 95% purity, and can custom manufacture concentrates of almost any other fatty acid.EPA fatty acid...
-
Product Disease Activity Models/Tissue Culture
MedPharm has developed proprietary models relevant for major disease areas such as psoriasis, atopic dermatitis, onychomycosis and continue to develop new ones through our dedicated innovation group. These sophisticated ex vivo experiments provide additional confidence to clients and their in...
-
Product Formulation of lyophilized products
A correct formulation means greater stability of the active ingredient and provides protection against the stress suffered during the lyophilization process itself.
The selection of the appropriate excipients is really important in the development of a freeze-dried product.
-
Product Certification of Suitability (CEP)
Book a private, one-to-one session with the Certification team to discuss issues such as the CEP procedure, CEP applications or inspections, please complete the online form and the team will be in contact regarding an appointment.
Please book using this url:
https://www.edqm...
-
Product SANAL® SQ Pharmaceutical Sodium Chloride (API)
SANAL® SQ Sodium Chloride Pharmaceutical Quality is our chemically pure product which is suitable for all pharmaceutical applications. Additionally this product is suitable for laboratory testing and chemical applications in manufacturing procees - This product comply with American Chemical Socie...
-
Product Analytical Techniques
Comprehensive analysis during formulation development and GMP manufacture is vital to ensure that your drug has optimal delivery properties and stability profile as well as supporting your regulatory submissions.
-
Product Regulatory Testing and Research based services for Pharmaceuticals, Chemicals, Phytochemicals, Herbal Formulations, Food and Medical devices
1) Analytical Testing Of Pharmaceuticals & Cosmetics2) Biotechnological Services
3) Microbiological Services
4) Bio - Compatibility Studies of Medical Devices(As per ISO 10993USFDA and MHLW Guideline)
5) Preclinical & Toxicological Services
6) Phytochemical & AYUSH Testing Se...
-
Product Method Development, Validation and Transfer
Kymos Pharma Services, S.L. offers wide range of chemistry, manufacturing & control analytical services in medicinal chemistry which includes method development, validation and transfer. Kymos has comprehensive knowledge in the development and validation of analytical proprietary and non-proprietary method...
-
Product Drug Product Development
We support our clients from early-stage research and preclinical phase to and beyond market approval by providing comprehensive development services, including
• Developability assessment • Pre-formulation screening • Formulation development • Lyophilization process development • Forced d...
-
Product SOFTGELS
At Softigel, we offer much more than traditional softgels, including everything from a single unit dosage form, to multiple delivery systems in a single dose. Softgels are an effective delivery system for oral drugs, especially those with low solubility and/or permeability (BCS Classes II, III and...
-
Product cGMP Manufacturing
1. Manufacturing facilities ensure: 1) Production flexibility at various scale 2) Total Production Capacity: 726 m3 including pilot production 3) Potent compound manufacturing (OEL Category 3A, CPT 1 Mug/m3) 2. Various Analytical Capabilities: 1) cGMP QC lab with HPLC, UPLC, GC, LC-MS, GC-MS, NMR, X...
-
Product Laser Micromachining Services & Systems
From pinholes in stainless steel discs to complex arrays in semiconductor guide plates, we provide a wide range of ultra-high precision laser drilling, cutting, milling, scribing and ablation contract services (and systems) for numerous applications in industry & academia. With over 45 years of experie...
-
Product Services - Lab analysis
We offer the quality standards that we apply to our own environment, machines, solutions and processes as services to our customers as well.
We make continuous investments in our specialist expertise. Quality assurance is the focus of our work. To ensure that we can meet your requirements r...
-
Product Pantoprazole Sodium
It is a digestive system medication suitable for the treatment of acute upper gastrointestinal bleeding such as duodenal ulcers, acute gastric mucosal lesions caused by gastric ulcers, and compound gastric ulcers.
-
Product QACS Pharmaceutical services
QACS Lab is GMP/GLP certified and provides contract laboratory testing for Pharmaceuticals. Methodologies stay in accordance with EMA & FDA. QACS is equipped with Microbiological - Chemical - Molecular - Packaging laboratory premises to provide variety of Pharmaceutical testing solutions &...
-
Product J.T.Baker® Viral Inactivation Solution
J.T.Baker® Viral Inactivation Solution Biotech Reagent is a highly effective, ready-to-use product, specifically developed for detergent-based viral inactivation in biopharmaceutical operations and plasma derived therapy processes. J.T.Baker® Viral Inactivation Solution is formulated with non-ionic deterge...
-
Product Analytical Services
Our Analytical Services encompass critical aspects of drug development and manufacturing, beginning with Analytical Testing to ensure product quality, employing advanced instrumentation and experienced teams to facilitate compliance. We excel in Method Development & Validation, utilizing HPLC and UHPLC...
-
Product Development of Formulation
Formulation Development- Cosmetic products
- Medical devices (skin and mucous membranes)
- Oral supplements
- Veterinary products
- Biocidal products
- Topical medicines
Stability tests - evaluation of the stability of cosmetic products, medical devices, food supplements and bioci...
-
Product Commercial Mass Spectrometry Services
Mass spectrometry is among the most powerful analytical techniques available for protein characterization. KBI’s state-of-the-art mass spectrometry core facility delivers unparalleled structural characterization services to our clients.
Our expert team brings decades of experience in protein and p...
-
Product Solid & Liquid Dose Drug Manufacturing & Development
From OTC and Rx to diagnostics and dietary supplements, Avéma manufactures a full range of solid and liquid dose products, all manufactured under strict FDA guidelines and cGMP compliance. With an ever-growing portfolio of innovative formulas and a diverse mix of state-of-the-art equipment, our offerings a...
-
Product Syringe and Vial Filling for Clinical and Commerical
Lifecore’s experts have gained the know-how to build processes around your requirements for smooth scale-up and high-quality manufacturing.
Whether we’re your primary partner or a secondary source for manufacturing, we ensure you're fully satisfied with the final produ...
-
Product Impurity Synthesis
Pharmaffiliates Analytics & Synthetics (P) Ltd, is an integrated CRO (Contract Reserach Organisition) established in year 2001. Presently our group consists of more than 130 Scientists with 3 R&D Centres offering its expertise in Custom Synthesis, Impurity synthesis, Isotoped lebelled compound...
-
Product Analysis Service
Lomapharm GmbH provides wide range of services including analysis service. It involves chemical and physical analysis of raw materials, intermediate and finished products according to national and international specifications as well as customer-specific information (microbiological purity, stability t...
-
Product VialArch
The Gasporox sensor module VialArch offers non-destructive Headspace Gas Analysis to be integrated directly onto the production line or into an inspection machine.
This unique laser-based solution is installed on the production line for 100% quality inspection and container closure ...
-
Product Analytical Development
BioDuro-Sundia’s Analytical Testing team offers high quality analytical services including method development and validation, qualification of reference standards, testing and release studies, stability studies, and CMC dossier preparation services. ...
-
Product Dissolution Testing - R&D services
For us dissolution testing is never a routine test but an essential tool in pharmaceutical formulation development to:
• evaluate the physicochemical properties of drug candidates to select the most appropriate solid form for further development (pre-formulation)
• compare pro...
-
Product Research & Development
Discovery - Biology, Lead Optimization, Libraries, Synthetic & Medicinal ChemistryDevelopment - Chemical Process R&D, Fermentation, Formulation Development, High Potency, Kilo Lab & Small Scale Manufacturing, Lipid Nanoparticle, Method Development/Material Science, Rare/Orphan, Separation Scien...
-
Product Highly Potent Pharmaceutical Principles
Olon relies on one of the most extensive expertise in handling highly potent pharmaceutical principles at global level, has raised the level of containment up to the actual standards and very large scale putting in place a set of primary and secondary containment designed to avoid the active substances...
-
Product Analytical Development and expertise
Our analytical experienced team support the pharmaceutical development during every phase of the development process. Analytical development transfer and validation, characterization capabilities, stability analysis, ICH Q3D (residuals solvent risk assesment), ICHQ3C (elemental impurities risk assesment...
-
Product Stability tests according to ICH
Neotron Pharma provides Stability tests according to ICH standards (zones I, II, IV). We can carry out stability studies in their entirety (storage + analysis) or provide only storage service. The conditions we can offer are listed below:
- 40°C ± 2°C , 75% ± 5% U.R.
- 30°C ± 2°C&n...
-
Product Analytical Services
Method development and phase appropriate validations to support clinical and commercial programsCompendial Testing
Raw Material Testing
-
Product Analytical Services - Inhaled Products
With extensive, state-of-the-art analytical testing facilities and equipment, our expert teams are able to develop and validate the methodologies required to characterise inhaled delivery platforms, especially DPI, pMDI and nebulised products.
To ensure seamless support for your development...
-
Product NETWORKING OF Excellence and innovation of the health sector. Made in France.
A team of experts serving the network.
POLEPHARMA stimulates industrial and economic development of actors of the French first pharmaceutical sector with actions that promote competitiveness, innovation and attractiveness to the advantage of the job market.

-
Product Quality Control
Our quality control department offers a broad range of services from incoming goods testing, in process controls to release of final product and stability testing. Our workflow ensures that all products are safe for market release and simultaneously functions as a feedback tool to help in the optimizat...
-
Product Hexane(HPLC grade)
Package size including 1L/bottle,2.5L/bottle,4L/bottle.Always keep 1000bottles in stock.
-
Product Analytical Services
ChemCon’s analytics team takes care of your inquiries if you are looking for ICH-compliant quality control, GMP validation, release analysis, impurity determination, reference standards or answers to other analytical queries. Close, outcome-oriented communication is the key to a successful partnership:...
-
Product Wasdell Manufacturing
Wasdell’s specialised pharmaceutical manufacturing facilities in the North East and the East Midlands are MHRA approved for the manufacture of non-sterile oral liquids with the capability and capacity to extend its services to other dosage forms. We can support the manufacture and packaging for cl...
-
Product Netpharmalab Consulting Services
Contract Research Laboratory. Analytical Services (physicochemical analysis, microbiological analysis and stability studies) and EU Import Lab.
-
Product Electron Beam
There is much to consider when choosing a sterilization technology. Sterigenics’ expert advisors look forward to working with you to understand and achieve your unique product goals. We offer all major sterilization technologies: Gamma Irradiation, Ethylene Oxide & Electron Beam as well as innovativ...
-
Product Analytical Services
From a drug product’s first raw materials to its release-ready batches, our experts rigorously evaluate and verify a product’s quality at key steps throughout the manufacturing process. Analytical services include material testing to verify product quality from the early stages of development, cGMP-com...
-
Product New product development
Delpharm group offers pharmaceutical services which includes new product development. It belongs to services and commitments category. It can support your development of sourcing the active ingredients in the product registration and monitoring of the file with the health authorities in providing you with ...
-
Product Analytical Services
Mabion offers a extensive suite of Analytical Services to ensure safety, efficacy and quality of your biologic product. Our portfolio includes development, optimization and validation of analytical methods, as well as stability studies, GMP release testing and in-depth drug characterization. In addition to...
-
Product CMC analytical support
Leveraging state-of-the-art technology and rigorous scientific methodologies, we provide a full spectrum of CMC analytical support, including:
· Method Development and Validation
· &nb...
-
Product Trace Metals Testing and Elemental Analysis
Our experienced analysts apply a strategic approach to sample preparation for difficult samples and a range of analytical technology, relevant to your needs, including inductively coupled plasma – mass spectrometry (ICP-MS) or inductively coupled plasma – optical emission spectroscopy (ICP-OES) or ion chro...
-
Product CONSULTING SERVICES ON PARTICLE CHARACTERIZATION
Consulting services which create value by combining our expert scientific background with highly qualified staff, equipments and facilities, to deliver advanced particle characterization studies such as:
- Development validation and transfer of analytical methods
- Comparative...
-
Product Outsourcing
Patheon by Thermo Fisher Scientific has a broad manufacturing platform for pharmaceutical and biologic products which provides sustainable solutions for mammalian cell-based and microbial-based manufacturing, green chemistry R&D and manufacturing technologies, and finished dosage production of biopharm...
-
Product Contract Manufacturing and Development, from Clinic to Commercial Scale
CoreRx provides comprehensive drug product pre-formulation, formulation, analytical, and GMP manufacturing and packaging solutions, enabling our partners to meet their drug program goals. CoreRx delivers these solutions from it’s development and manufacturing campus in Clearwater, Florida and it’s...
-
Product Licensing for Partners
PharmaMatch represents companies from all over the world and we have always shown ourselves to be a reliable and trustworthy partner. Our sales team has an excellent network and they are continually assessing the market per product to find the best match. Our partners can expect our Sales team to appro...
-
Product Analytical Testing
Analysis of active ingredients and their characterization, during and after manufacturing, to ensure the integrity of the finished product.
Laboratoires Chemineau provides a wide range of services which includes control & release. Features: it includes complete equipment up-to-date HPLC/ CPG /...
-
Product Research & Development Capabilities
PHT International Inc. offers a wide range of services which includes research & development capabilities. Features: it includes process development, process improvement, sample repackaging, sample production, sample analysis. Contact us for more information.
-
Product Packaging and Supply Chain Solutions
Tjoapack's packaging and supply chain solutions help our customers to make the world a healthier place. Our expert team work to facilitate your life sciences research, production and the delivery of medicines to patients across the globe.
We offer: - Packaging solutions for; oral solid blisters a...
-
Product Analytical Services
Mefar offers wide range of analytical services which includes process validation / scale up batches / stability studies / analytical method transfers and more.
Please contact us for more information.
-
Product Raw Material Services
Supply of Active Pharmaceutical Ingredients (APIs) and excipients to pharmaceutical manufacturers
Services include:
Purchase of active pharmaceutical substances, excipients, primary packaging materials and reagents Analytical services to ensure identity and compliance
I...
-
Product Laboratory & Analytics
Samples, bulk products or drugs: Our experts analyze your product and evaluate whether it meets all individual and regulatory requirements.
Among others our modern laboratory is equipped with:
• HPLC-systems • Dissolution testing • UV/Vis- and atomic absorption spec...
-
Product Biologics Characterization
SGS’ range of services dedicated to biopharmaceutical product characterization bring the most recent developments in testing technology to companies across the globe. In 2010, SGS acquired the M-Scan Group, the world leaders in the application of advanced mass spectrometry
techniques for protein and c...
-
Product Physico-Chemical. Analysis
Physico-chemical methods are required in a range of products and compounds such as excipients, API’s, bulk material and finished products. Product characterization methods are often mandatory requirements within compendial monographs e.g Ph.Eur, USP, JP, BP. etc. as well as individual Marketi...
-
Product Transfer Methods Service
Avara Pharmaceutical Services offers development support services, including transfer methods. Contact us for more information.
-
Product Thermal Analysis
PolyCrystalLine offers a wide range of analytical services which includes thermal analysis. Its an indicator of conformational stability, reaction-rates and composition of the sample. They are also useful for determining sample purity and water, carbonate and organic contents and for studying decomposition...
-
Product Manufacturing Process Services
Pharma-Data sa offers wide range of services which includes manufacturing process services. It includes sourcing of the appropriate drug product manufacturer (cmo), manufacturing process development and validation, coordination of gmp auditing and approval by eu and fda. Contact us for more information.
-
Product Laboratory Excellence
We provide the expertise and counseling you need to secure the best lab performances and avoid recalls and non-compliances.Don’t risk losing quality and trust: choose the right training, guidance and support.
-
Product Raw Materials Testing Services, Compendial
Pace analytical life sciences llc offers wide range of services which includes raw materials testing service. It provides raw material testing services to the pharmaceutical, biopharmaceutical and medical device industries. It offer excipient testing to support usp/nf, ep, bp, jp, fcc and acs monograph tes...
-
Product Chemical and Material Testing
Alliance Technologies is a DEA licensed and FDA registered contract chemical testing laboratory and consultancy. Our senior analytical chemists provide a wide range of analysis and material testing services to a diverse, international client base with expertise in problem solving, method development, fail...
-
Product Microbiology
Excerpt from the range of services offered by our microbiological department: • microbial contamination testing (bioburden) • identification of microorganisms • sterility testing • biological indicator testing • bacterial endotoxin testing (LAL), rFC testing
• assay of antibiotics • effi...
-
Product Analytics
Excerpt from the range of services offered by our analytical department: • physico-chemical testing • chromatography • spectroscopy/spectrometry • determination of TOC/TNb (water analysis/cleaning validation) • particle measurement
• elemental analysis
• dissolution testing
• pho...
-
Product Partner laboratories
BioChem Labor für biologische und chemische Analytik GmbH offers a wide range of services which includes partner laboratories. Features: Identification of impurities, extractables / leachables, test for pyrogens in rabbits according to Ph Eur.. 2.6.8 / USP, test for abnormal toxicity Ph. Eur. 2.6.9 / USP, ...
-
Product Mérieux NutriSciences | Pharma & HealthCare complete services
Download our brochure: https://bit.ly/4dzryoA
Our complete range of Pharma services include: • Quality controls • Investigation studies • Absorption studies • R&D and validation activities • Stability & storage • Cleaning and disinfectants validation • Environmental monitoring
-
Product Merieux NutriSciences | Absorption and efficacy in vitro studies
Download our brochure: https://bit.ly/4dzryoA
In vitro models mimicking different biological barriers can be adopted to perform absorption studies and evaluate efficacy of products, ingredients or new formulations before to proceed with the next step evaluation (e.g. clinical trial).
q...
-
Product Merieux NutriSciences | Nitrosamines analysis on pharmaceutical products
Download our brochure: https://bit.ly/4dzryoA
Thanks to the long-standing experience, Mérieux NutriSciences has been developing various strategies and approaches for the determination of nitrosamines residues in different matrices through sophisticated mass spectrometry combined with a pool of ex...
-
Product Merieux NutriSciences | Extractables and Leachables studies
Download our brochure: https://bit.ly/4dzryoA
Pharmaceutical packaging can release chemicals into the drug product that can not only impair its effectiveness, but also be harmful to the patient. Similarly, medical devices can undergo leaching processes during their use that could negatively affec...
-
Product Merieux NutriSciences | Medical devices services
Download our brochure: https://bit.ly/4dzryoA
Thanks to a valuable pool of experts, Mérieux NutriSciences supports you in the development of the testing plan to determine which studies are necessary to ensure that the device is safe and effective, meeting the essential requirements for affi...
-
Product API Services, Chemical Development & Manufacture
Almac’s strength in API development and manufacture is proven by being the partner of choice for many pharma and biotech companies seeking integrated drug development solutions from molecule to market.
Our technical expertise and extensive facilities enable us to offer integrated API contract ma...
-
Product 14C Radiolabelling
Almac has extensive experience in the synthesis and analysis of stable (non radio-active) and 14C isotope labelled compounds – from drug discovery through to launch – we can label any compound at any stage, including small molecules, peptides and larger bio molecules.
Our custom radiolabell...
-
Product Physical Sciences - Preformulation & Solid State Services
Almac's Physical Sciences groups' comprehensive range of services and expertise will help strengthen the success of your product. Identifying and consistently producing a drug in its optimal physical form is vital to any successful drug development programme. It also avoids the need for bridging toxicologi...
-
Product mRNA Characterisation and Analysis
Characterization and analysis of mRNA supporting vaccine and therapeutic development, including assessing clinical translation efficiency and immunogenicity. At our GLP / GCP / GMP laboratories, scientists can test a mRNA drug substance or drug product to help you confidently assess batch to batch manufa...
-
Product Gene Therapy Characterisation and Release Testing
Characterization and release testing of gene therapies: supporting CMC requirements for IND applications and commercial release through expert analysis and stability testing. We provide method development and validation to meet your milestone and regulatory requirement and help you to confirm identity a...
-
Product Vaccine Characterization and Bioanalytical Support
Our vaccines development experts provide a suite of services supporting the analysis and quality control of process and batch release samples and stability studies. We have support the development of a range of vaccines including mRNA, protein, glycoprotein, DNA, carbohydrate, lipopolysaccharide, lipi...
-
Product Elemental Impurity Testing ICH Q3D
Testing for elemental impurities ICH Q3D: To successfully implement ICH Q3D requirements, our experts in elemental impurities and toxicology can help you develop a compliance strategy. We can provide screening studies and data to aid risk assessment, if this does not already exist, or develop and valida...
-
Product Nitrosamine Impurity Testing
Nitrosamine - testing for its impurities: Nitrosamine impurities such as N-nitrosodimethylamine (NDMA) continue to be an area for concern as highlighted by the US FDA and other regulatory authorities. At Intertek we believe flexible solutions are the way forward to overcome technical challenges and ac...
-
Product Liposomal Drug Delivery Technologies Development Support and Analysis
Liposomal drug product characterisation according to the FDA CMC guidance to support your new drug application or biologics license applications. Including:
• Physicochemical parameters determined to be CQAs (e.g. particle size, size distribution, zeta-potential and physical stabi...
-
Product Liquid, cream & gel forms physical characterization services
cGMP services include:- Particle size by laser diffraction on sprays/aerosols and liquid dispersions
- Particle size, shape & Chemical identification in gels, creams, liquids by MDRS
- Thermal analysis DSC
- colloids, liposomes and nanoparticle size distribution and zetapotential by DLS ...
-
Product EVs extracellular vesicles characterization services
Alfatestlab is equipped with the latest technology platform to provide rapid, reliable and accurate characterization of EVs following MISEV 2023 guidelines. Based on Nanoparticle Tracking Analysis (NTA - Nanosight) and Single-Vescicle Multiparametric Analysis (Leprechaun), our services for EVs of...
-
Product Aerosol & Spray particle size distribution analytical services
We provide cGMP analysis of sprays and aerosols: droplets particle size distribution over time of sprays and aerosols, using laser diffrcation technique (Spraytec by Malvern Panalytical)
-
Product LNPs production services by microfluidics
We offer method development services for the production of lipid nanoparticles LNPs using the microfluidics platform Sunshine from Unchained Labs.

-
Product Lyophilization Process Development (Quality By Design approach)
With the aim of obtaining a product that meets the critical quality attributes, but with the most optimized freeze-drying process.
Our focus is the total control of the process. We develop freeze-drying processes in a previously defined design space: Quality By Design approach.
-
Product European Pharmacopoeia Supplements 11.6 to 11.8
The 2025 subscriptions to the European Pharmacopoeia (Ph. Eur.), including the Supplements 11.6 to 11.8, are now available for purchase in the webstore. Two subscription formats are available: print and online versions.
-
Product Nitrosamine Detection
We provide Method Development and Method Validation for new analytical methods or Method Transfer for Routine Testing according to existing validated methods for raw materials and final products. QACS is GMP/GLP certified.
• performed with UPL...
-
Product Leachables Extractables
QACS tests for organic and inorganic components, Volatile, Nonvolatile residues and perform Elemental impurities analysis. Packaging Migration/Compatibility studies are also provided. Studies are performed with state of the art equipment.
▶️ info@qacslab.com +30 21...
-
Product Stability Studies
QACS Labs offer drug development services. Pharmaceutical testing includes pharmaceutical stability testing, storage stability services and custom solutions based on international standards. We test API’s, raw materials and final products in recommended environmental storage conditions such as Temperature,...
-
Product Method Validation & Transfer
Pharmaceutical testing services during and after drug development from QACS ensure GMP compliance and product safety. Method validation studies aim to demonstrate analytical suitability of procedures according to their intended use/purpose. QACS transfer method protocols ensure, that results generated by s...
-
Product J.T.Baker® Direct Dispense packaging system
Enhance the effectiveness of your products and streamline your biopharmaceutical manufacturing processes with the J.T.Baker® Direct Dispense packaging system. This novel packaging platform makes it easy to deliver powdered performance materials and excipients directly to your process, whether you are using...
-
Product Avantor OmniTop Sample Tubes® adjustable volume sampling system (AVSS)
AVSS helps our customers overcome complex scale-up challenges, particularly in sensitive applications like cell and gene therapy production and mAbs downstream processing. The single-use sampling device, with reusable adjustment tool and rack, can easily be used stand alone or configured in a manifold ...
-
Product Avantor® Magnetic Mixing System
The Magnetic Mixing Bag offers a sterile, single-use mixing container designed to maximise mixing performance while minimising disruptive shear. The 5-bladed impeller is floating freely due to magnetic forces minimising risk of particle generation and ensuring a safe and homogenous mixing of product. A...
-
Product MasterSense™ Smart Peristaltic Pumps
NEW integrated sensor technology, intuitive touchscreen and MasterflexLive® remote monitoring for the biopharma industry of tomorrow
-
Product Core Technologies and Services
• API / GMP Manufacturing • Rapid Process Development, Flawless Upscaling, and Economy of Scale-Production • Simulated-Moving Bed (SMB) Chromatography • Heterocyclic, Hazardous and Malodorous Chemistries • Organometallic and Cryogenic Chemistry • Transition-Metal Catalysis • High-Pressur...
-
Product Reference Standards
Pharmaffiliates is Supplying all the Pharmacopoeial Reference Standards (i.e. USP, EP, BP, IP, JP).Apart from this we offer Pesticide Standards, Phyto-chemical Standards, Food & Environmental Standards, etc.
For more details please send your enquiry on marketing@pharmaffiliates.com
-
Product Bioanalytics/Molecular Biology
Excerpt from the range of services offered by our bioanalytical/molecular biological department:
• determination of mycoplasma • biological contamination testing • determination of content and identity of nucleic acids • determination of biological activity
• protein/antibo...
-
Product Recipharm Analytical Solutions™
Through Recipharm Analytical Solutions™, we support customers with stand-alone analytical requirements. Our analytical development team has experience from developing hundreds of analytical methods every year, supporting development of formulations ranging from powder in capsules and IV solutions to ER tab...
-
Product Protein Analysis and Charactersation
Our protein analysis scientists provide characterization services in accordance with the ICH Q6B Guidance to ensure the quality and consistency of your product. With strengths in protein structure analysis including higher order structure, physiochemical property determination, bio...
-
Product Isolation and Characterization of Product-related Impurities
Intertek offers product-related impurity analysis in line with ICH Q6B with laboratory-scale isolation and a range of chromatography or mass spectrometry approaches. Our experienced scientists perform detailed characterization using a diverse range of technologies which include MALDI-MS, LC-MSMS, HPLC...
-
Product Quantitative Immunoassays
Intertek offers quantitative immunoassays such as ligand binding assays for toxicokinetic and pharmacokinetic studies supporting biologic preclinical and clinical development. Our capabilities for quantitative immunoassays include developing methods for biologics and biosimilars, method transfer, opti...
-
Product Immunogenicity Assays
Intertek offers immunogenicity assays for detection of anti-drug antibodies (ADAs) and neutralizing antibodies (NAbs) in support of nonclinical and clinical studies. Our immunogenicity assay experts utilize a multi-tiered approach to measure ADAs and NAbs, and are experienced in the development and va...
-
Product Ligand-binding Assays
Intertek offers wide range of pharmaceutical services which includes ligand-binding assays. It belongs to immunochemistry services category. It includes elisa, electrochemiluminescence (ecl), radioimmunoassay (ria), etc.
To learn more, visit our website:
www.intertek.com/pharmaceut...
-
Product Viral Vector Characterisation and Release Testing
Viral vector characterization and release testing services from Intertek's centre of Excellence for Biologics Characterisation, help you to establish and address critical quality attributes (CQAs) that impact product safety, purity, and potency. We deliver robust analytical assays to assess vector pro...
-
Product Residual DNA Testing for Cell and Gene Therapies
Our scientists use Real-time qPCR and digital droplet PCR (ddPCR) techniques for robust quantification to support process validation, monitor batch to batch variation and support of GMP lot release, helping to ensure that products produced from a range of common host cell lines (HEK293, E.coli an...
-
Product ATMP Analytical Development Services
Advanced therapy medicinal product (ATMP) analytical services & chemistry, manufacturing, and control (CMC) support including characterisation, stability and release testing. Our GLP / GCP / GMP laboratories have supported developers and manufacturers for over 20 years through the provision of adv...
-
Product Analytical research & development
Our analytical experts know what it takes to bring a pharmaceutical product to the market, from discovery, feasibility to GMP. Having worked with more than 100 unique molecules and even more formulations, we may state that we have faced the most difficult CMC challenges that are involved.
...
-
Product Pharmaceutical Deformulation / Reverse Engineering
Our experience in excipient characterization and deformulation of pharmaceutical formulations gives us the opportunity not only to identify and quantify individual excipients in a complex pharmaceutical formulation, but often also confirm the excipient grade, excipient quality and sometimes even the excipi...
-
Product Excipient Quality Testing and Selection Services
Excipient testing, composition and variabilityExcipients are either natural / naturally derived or synthetic / semi-synthetic. In all cases the exipients are obtained through chemical processing of a raw material source that usually has an animal, vegetable or mineral origin.
...
-
Product Excipients Formulation Development Support
Excipients play a central role in the drug development process, in the formulation of stable dosage forms and in their administration. Though excipients were at one time assumed to be “inactive” ingredients, it is now understood that excipients can have an important impact on the pharmaceutical...
-
Product Fermentation
Relying on an experience gained over more than 50 years, OLON represent one of the most extensive know how of microbial fermentation in Europe. The Group, global leader in biomanufacturing, has two Biotechnology Centres located in Italy and is one of the first companies in Italy producing via microbial ...
-
Product Research & Development
Avéma offers formulation, process and analytical development support of new or existing products using the most current and effective active ingredients and delivery systems. Our team of scientists bring decades of pre-formulation development expertise to your products, combined with the knowledge of how t...
-
Product Stability Studies
Kymos Pharma Services, S.L. offers wide range of chemistry, manufacturing & control analytical services in medicinal chemistry which includes stability studies. Kymos provide full service of ich and on-going stability programs for third parties. The services, at client's choice, can include: method tra...
-
Product Polysaccharides analysis
Kymos S.L. offers wide range of chemistry, manufacturing & control analytical services in medicinal chemistry which includes polysaccharides analysis. Kymos have implemented and validated these challenging assays according to usp monograph and are ready to be used for drug product and drug substance. H...
-
Product Bioanalysis of Oligonucleotides
We quantify according to GLP nucleic acids in various biological matrices for you. We develop standardized and reproducible methods that will bring you through FDA/EMA registration. No matter which nucleic acids you develop as therapeutics, biomarkers or similar, we offer you the quantification of your nuc...
-
Product Pharmacopea Analysis
Neotron SpA provides wide range of analytical control according to EP, USP, JP, BP, CHP.
Contact us to ask for tests in relation to your monograph of interest.
-
Product R&D service
Neotron Pharma is able to offer you a research and development service. The research and development laboratory, operating under the ISO regime, will be able to develop customized methods for the customer in faster times and with lower costs. Subsequently, the customer will be able to validate the GMP meth...
-
Product Method Validation
Neotron Pharma is able to validate analytical methods developed internally or transmitted by the customer using top of the range equipment such as:
- HPLC-UV / DAD / MSMS
- GC-FID / ECD / MSMS (even in Headspace)
- ICP-MS / AAS / AES / OES
- Post-column derivatization
- Particle si...
-
Product Elemental Impurities
In light of the growing interest of the pharmaceutical world on the issue of determining Elemental Impurities in accordance with tables 1, 2a, 2b and 3 of the ICHQ3D, Neotron Pharma laboratory, thanks to its decades of experience in the analysis of metals, has developed a series of analytical proposals to ...
-
Product Contaminants
Neotron Pharma, thanks to its many years of experience in the Food department, is able to quantify the presence of contaminants such as Pesticides, Aflatoxins, Mycotoxins under GMP regime. This type of analysis can be fundamental for all those suppliers of raw materials and Erbal intended for the supplemen...
-
Product Residual solvents
Neotron Pharma is able to perform screening tests in accordance with USP <467> for solvent classes 1,2,3, validation activities for methods transmitted by the customer or to develop customized methods for particular solvents.

-
Product Extractables & Leachables
There are many contaminants that could be released inside a drug during the production process or by contact with the packaging material. Neotron Pharma will be able to support you from the study of Extractables, to the toxicological evaluation up to the control of the Leachables. What distinguishes us is ...
-
Product Nitrosamine impurities
Neotron Pharma, on request of several customers, has provided an effective methodological approach for Nitrosamine alerts management to support the pharmaceutical industry. Currently the laboratory is able to perform screening and validation activities for more than 11 Nitrosamine residues in API, FP and e...
-
Product Pyrrolizidine Alkaloids
Neotron Pharma has an HPLC-MSMS method for the determination of 28 pyrrolizidine alkaloids on different matrices. We currently collaborate with numerous herbals producers for these routine checks for both the Pharma and Food markets
-
Product Analytical development
Alcami offers a fully-integrated analytical method development, method validation, and testing solution with a full complement of advanced analytical and information technologies. From Alcami’s electronic laboratory notebook (ELN) data collection system to its integration with our formulation development, ...
-
Product Microbiology
Microbiological testing is an important factor in ensuring your product’s safety, efficacy, and timely project completion. At Alcami, we combine decades of microbiological expertise, developed from serving the biotech, pharmaceutical, and medical device industries, to provide the most current and effective...
-
Product Integrated offerings
Alcami is a Us-based, contract development, testing, and manufacturing organization for pharma and biotech companies. Our goal is to support our clients in making their projects go from potential to reality day-after-day.
Core Capabilities:Sterile-fill finish development and manufacturingO...
-
Product Residual Solvents and OVI Testing
We provide expert determination and identification of residual solvents in pharmaceutical articles, helping customers ensure that residual solvents have been reduced to acceptable levels in drug products, drug substances and excipients. Often it is prudent to ensure residual solvents are controlled for all...
-
Product X-ray powder diffraction (GLP & GMP) for pharmaceuticals
Intertek offers wide range of pharmaceutical services which includes x-ray powder diffraction (glp & gmp) for pharmaceuticals. It belongs to pharmaceutical analysis services category. It includes gmp services, stability and pharmaceutical testing, physical characterization techniques for pharmaceutical...
-
Product Total protein quantification
Intertek offers wide range of pharmaceutical services which includes total protein quantification. It belongs to biopharmaceutical protein analysis services category. It includes amino acid analysis, absorbance at 280nm. Lowry assay, bradford assay, bca assay, etc.
-
Product Method validation
Intertek offers wide range of pharmaceutical services which includes method validation. It belongs to bioanalytical services for preclinical and clinical studies category. Contact us for more information.
-
Product Medical Device Testing
R&D Analytical SupportIntertek’s significant expertise in bioanalysis and clinical pharmacokinetic studies allows us to support medical device companies working in the areas of preclinical and clinical drug development. Our support services include assay development for API’s, impurities, trace metals ...
-
Product Analysis of Inhalation drug products
Kymos Pharma Services, S.L. offers wide range of chemistry, manufacturing & control analytical services in medicinal chemistry which includes analysis of inhalation drug products. The devices used for inhaled and nasal drug delivery are collectively referred to as orally inhaled and nasal drug products...
-
Product Extractables & Leachables
Kymos Pharma Services, S.L. offers wide range of chemistry, manufacturing & control analytical services in medicinal chemistry which includes extractables & leachables. In recent years, the pharmaceutical industry has developed a better understanding of the impact of extractables and leachables on ...
-
Product Characterization of Biologics and Biosimilars
Kymos Pharma Services, S.L. offers wide range of chemistry, manufacturing & control analytical services in medicinal chemistry which includes characterization of biologics and biosimilars. It includes range of biologics such as: hormones and toxins, biosimilars (proteins and monoclonal antibodies), inn...
-
Product Analytical Chemistry
Kymos Pharma Services, S.L. offers wide range of chemistry, manufacturing & control analytical services in medicinal chemistry which includes analytical chemistry. Kymos has wide experience in performing analytical tests of raw materials, apis, intermediate products, finished products, packaging materi...
-
Product Microbiology
Kymos Pharma Services, S.L. offers wide range of chemistry, manufacturing & control analytical services in medicinal chemistry which includes microbiology. Kymos provide microbiological testing for sterile and non-sterile apis and drug products. A fully-equipped laboratory is available including a clea...
-
Product Microbiology ISO testing
Kymos Pharma Services, S.L. offers wide range of chemistry, manufacturing & control analytical services in medicinal chemistry which includes microbiology iso testing. Kymos provide microbiological testing for nutraceutical and cosmetic products. A fully-equipped laboratory is available including a cle...
-
Product Batch Testing & Batch Release
Kymos Pharma Services, S.L. offers wide range of chemistry, manufacturing & control analytical services in medicinal chemistry which includes batch testing & batch release. Features: apis certificate of analysis according to the relevant pharmacopoeias, individual parameter determination to be incl...
-
Product Elemental Impurities
Kymos Pharma Services, S.L. offers wide range of chemistry, manufacturing & control analytical services in medicinal chemistry which includes Elemental Impurities. We provide Elemental Impurities testing using ICP-MS according to ICH Q3D and European Pharmacopoei...
-
Product Vaccine Potency Assays
Kymos Pharma Services, S.L. offers wide range of chemistry, manufacturing & control analytical services in medicinal chemistry which includes vaccine potency assays. Suports in the development of potency assay of vaccines for quality control in order to substitute "in vivo" testing. We are able to perf...
-
Product GMP and CMC Laboratory Services
We provide regulatory-driven, phase-appropriate laboratory services in support of CMC programs seeing you through pre-formulation, formulation and product release. Our capabilities include centers of excellence for method development and validation, analysis, stability studies,...
-
Product Stability Testing
With a network of ICH stability storage facilities in the UK, US and Australia, we offer an extensive capacity and a range of conditions including climatic walk-in chambers, cabinets and refrigerated as well as freezer storage, covering all ICH stability conditions. These are fully controlle...
-
Product Inhalation Drug Product Development Services
Our pharmaceutical auditing and management services give you a transparent view of your supply chain enabling you to identify and mitigate the intrinsic risks in your operations, supply chains and business processes. Through our shared audit programs, delivered by our global network of specialist ...
-
Product Extractables and Leachables Testing
Our experts have over 30 years of experience in specialized analytical and toxicology assessment for extractable leachable testing. Intertek offers extractables and leachables testing services through GMP-compliant laboratories located in Whitehouse, NJ (USA) and Basel (Switzerland) wit...
-
Product Fine Chemicals
SPECIAL TECHNOLOGIES AND REACTIONS
Special Technologies
• High-pressure reactions up to 64 bar: H2, CO, NH3, Amines, CO2 • Cryogenic: -80°C • Supercritical Fluid Chromatography (SFC), Simulated Moving Bed Chromatography (SMB) • Ultra low vacuum distillation • ...
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product Sterile drug product CDMO services
Thermo Fisher Scientific's flexible aseptic manufacturing and sterile fill finish solutions for your molecule’s unique needs and challenges will enable success in early development, late-phase, and commercial manufacturing.
Thermo Fisher offers extensive sterile product development and commercial ...
-
Product Regulatory Support
Our experienced regulatory team has a good understanding of the regulatory framework in every region. We’ve obtained registrations globally through our clients. Our team provides clients with quality dossiers compiled in accordance with the latest guidelines, which result in numerous successful registr...
-
Product API Sourcing
PharmaMatch has an extensive network of API Active Pharmaceutical Ingredients (API) suppliers, ensuring our clients are matched with the right partner based on quality, price and regular supply. We can also provide third party audits or pre-audits for our clients to ensure the facilities are up to st...
-
Product Contract Manufacturing Organisations
PharmaMatch has global contacts with contract manufacturing organisations (CMO’s). These state of the art CMOs are EU and/or US approved and follow the GMP standards of production. As clients demand high quality standards and a reliable supply chain with the best commercial conditions, the CMO par...
-
Product Lab Services
Through one of our partners, we can provide comprehensive analytical support during the development of drug formulations. By combining innovative approaches, modern instruments and advanced knowledge in the field of analytical science we are able to achieve high performance in terms of selectivi...
-
Product Pharma Materials Physical Characterisation
Our solid state characterisation teams support pharmaceutical product development during through API solid state characterisation packages to determine particle shape and size distribution, surface area, porosity, moisture or solvent content, solubility, pH, solid state stability, solution stability and th...
-
Product Narcotic Substances
Neotron Pharma is able to perform all the analytical techniques requested by the customer on APIs or finished products subject to restrictions as they are part of the narcotic substances. The laboratory will be able to verify if your specific active ingredient is free to manipulate or subject to restrictio...
-
Product Laboratory Services
Curida offers Lab services1 chemical lab
3 microbiological labs, including sterility lab and lab for test organisms
Main services:
Quality Control
Method development and validation
Stability testing
Contract laboratory
-
Product Cleaning Validation
Kymos Pharma Services, S.L. offers wide range of chemistry, manufacturing & control analytical services in medicinal chemistry which includes cleaning validation. It is a relevant gmp activity to prevent contamination of biopharmaceutical products by ensuring that the processing equipment is suitable f...
-
Product Instrument-Based Analytics
The pharma laboratories within the Tentamus Group are equipped with state of the art analytical instruments offering an unparalleled array of analytical and bioanalytical services. The range covers simple Pharmacopeia methods (e.g. Ph.Eur, USP) to very complex analyses of biomolecules. We offer all kin...
-
Product Microbiology
Microbiological tests are mandatory for non-sterile and sterile pharmaceutical products as well as medical devices. Tentamus Pharma Labs provide all standard test methods in this field and support your products in development or commercial phases. For non-sterile products, microbial enumeration tests (...
-
Product Pharmaceutical Products
Bringing a pharmaceutical product to the market entails many complex, time consuming and expensive activities. Analytical expertise is a cornerstone in this development process. Tentamus Pharma Labs can support our customers throught the entirety of the development process and commercialization by prov...
-
Product Medical Devices
The EU Medical Device Regulation (MDR) requires a more stringent characterization of products in the Medical Device field. The MDR is madatory for all new products and existing products have to comply by May 26th 2020. Tentamus Pharma Labs are equipped to support you in fulfilling MDR requirements. Bio...
-
Product Biomolecules and ATMPs
Biomolecules like antibodies have revolutionized the pharmaceutical industry during the last decade and new products (e.g. Advanced Therapy Medicinal Products – ATMPs) are evolving in clinical studies and on the market. Tentamus Pharma Labs are among the leaders of analytical support for these product ...
-
Product Medical Cannabis
Medical Cannabis is a rising star in the pharmaceutical arena. Research indicates, that it might open new ways in alleviating and treating severe conditions in a variety of indications. One of the biggest challenges for companies in the field are regulatory and analytical requirements of the pharmaceut...
-
Product Product Release Testing Service
Avara Pharmaceutical Services offers development support services, including product release testing. Contact us for more information.
-
Product Quality Support
We ensure that batch documentation delivered to clients is timely, complete and compliant with current good manufacturing practices (GMP) requirements and approved MA. Our Quality Assurance (QA) experts closely cooperate with production and QA teams at CMOs to coordinate change control, deviation, com...
-
Product Supply chain
Our experienced team, based in both the Netherlands and in India, ensures a hands-on approach in achieving the best possible supply chain opportunities for our clients. This is not limited to timely deliveries; checking the prices and availability of the API is also part of our core activities. ...
-
Product Structural Characterization
PolyCrystalLine offers a wide range of analytical services which includes structural characterization. Its an characteristics of the unit cell can be used to predict the morphology and physical properties of a drug, provides phase identification of a crystalline material and information on unit cell dimens...
-
Product Physical Characterization
PolyCrystalLine offers a wide range of analytical services which includes physical characterization. Its an physicochemical properties of a compound is essential so that the formulation process can be rational and streamlined. Contact us for more information.
-
Product Cgmp Quality Control
PolyCrystalLine offers a wide range of analytical services which includes cgmp quality control. Its provide a full complement of state-of-the-art gmp analytical testing resources and expertise to support cgmp quality control testing for raw materials, active pharmaceutical ingredients (api) and intermediat...
-
Product Method Development & Validation
PolyCrystalLine offers a wide range of analytical services which includes method development & validation. It can be entrusted with the most sensitive projects for method development and validation of api’s intermediates, raw materials and finished products. Contact us for more information.
-
Product Analytical Development And Validation Services
Pharma-Data sa offers wide range of services which includes analytical development and validation services. It includes justification of specifications, drug product release testing, method transfer, validation of microbiological methods (sterility, bet etc), filter validation (for sterile products – leach...
-
Product Stability Testing Service
Pharma-Data sa offers wide range of services which includes stability testing service. According to ich guidelines covering zone iii and iv photo stability. Contact us for more information.
-
Product Dossier Writing Service
Pharma-Data sa offers wide range of services which includes dossier writing service. It includes ectd dossier preparation, dcp/mrp submission and follow up, life cycle dossier management. Contact us for more information.
-
Product Pharmaceutical / Biopharmaceutical Testing Services
Pace analytical life sciences llc offers wide range of services which includes product release testing for pharmaceuticals service. It provides pharmaceutical product release testing for both qa pharmaceutical release testing and clinical release testing. It offer quality assurance/quality control product ...
-
Product One-stop Test Services for Cosmetics
With proven global expertise in cosmetic testing, auditing and certification, CD Formulation offers a variety of services to help you check the safety, quality and performance of your products and systems, and protect your brand. We help you verify that your cosmetics and personal care products c...
-
Product Narrow Mouth Bottles
PP, LDPE & HDPE with PP closure. Leakproof & Autoclavable. Exellent chemical resistance to most acids, bases and alcohols. Material conforming to USP Class VI. Autoclavable with or without contents. Contents are easily visible. Ideal for storing, packaging and shipping liquids.
-
Product Amber Narrow Mouth Bottles
Amber Narrow Mouth Bottles: Material conforming to USP Class VI. Leakproof. Protects light-sensitive liquids. Rigid and exellent chemical resistance to most acids, bases and alcohols. Ideal for storing, packaging and shipping liquids. Good for freezer storage to -100° C .
-
Product Sterile Erlenmeyer Flask
Sterile Erlenmeyer Flask. Flat base & Baffled base. PETG with HDPE closure. Vented & Non-Vented. Material conforming to USP Class VI. Non-cytotoxic and Free of detecable pyrogens. Graduated. Individual packed. Ideal for suspension cell culture.
-
Product Preclinical Laboratory Services
MYER's team counts with 30+ years of experience conducting nonclinical efficacy in vitro and in vivo experiments. Small molecules, biologics, devices, diagnostics. Contact us to get a quote!
-
Product Nitrosamines - QC / Development / Validation
LC-MS/MS methods are nowadays commonly used for nitrosamines analysis in all types of materials, since it allows analyte-specific detection based on both retention time and structurally specific fragmentation information in conjunction with high sensitivity.
After the establishment of the ni...
-
Product Analytical Services
Physico-Chemical testing
- General identification (TLC, HPLC)
- Assay (UV/VIS, HPLC-UV, GC-FID, LC-MS/MS) and dosage uniformity
- Related substances identification and quantification (HPLC-UV, GC-FID, LC-MS/MS)
- Physical determinations (pH, viscosity, density)
- Moisture...
-
Product Analyses of your chitin, chitosan or chitosan derivatives
Do you need further analyses for your ordered products? We would also love to analyse your own chitin, chitosan or chitosan derivative.
In addition to our standard analyses like degree of deacetylation, viscosity, ash content and content of heavy metals, HMC+ offers different other analyses. ...
-
Product Quality control laboratory
Batch testing and GMP EU batch release for commertialization of pharmaceutical products within the European market.
Import and batch certification
Physicochemical analysis: • General Identification Test (UV, IR, TLC, HPLC, colourants) • Physical Tests (pH, viscosity, hardness, disintegrati...
-
Product In Vitro Dissolution and Release Testing - R&D Services
Support formulation development with our experience in drug release testing. We offer a range of methods to:
• Evaluate the physicochemical properties of drug candidates to select the most suitable dosage form for further development • Elucidate drug release mechanisms, such as diffusion, erosion, etc...
-
Product Analytical Testing
Glatt Pharmaceutical Services develops and produces solid pharmaceutical dosage forms. Our focus lies on multiparticulate systems such as pellets, micropellets and granules. Whether you are looking for optimal bioavailablity or taste masking, improved solubility or stabilization of the dosage form, we have...
-
Product Analysis of biocides for registration in BPR
Analysis of active ingredients and impurities.Accelerated and long-term storage 5-patch analysis
-
Product Analysis of coatings of medicine products
Analysis of EG silver coated products
e.g. implant meshes,
nails and discs, wound dressing,
-
Product Analitycal Method Development
• Method development, optimization, validation and verification. • High skill operating with HPLC/UPLC • Cost and time focused • Working according to GLP standards • R&D Support

-
Product Other analytical and related services
• Stability Studies Design and Execution (chambers available) • Probiotic testing • Degradation studies • Animal Studies • Cell Research • Market Research • Supplier Qualification

-
Product Precious Metal Recovery Service
Mastermelt recover precious metal from heterogenous and homogeneous catalyst waste streams. We specialise in difficult material to find new routes to recover metal from complex waste streams.

Looking to outsource analytical pharma studies? Find 100+ global CDMOs offering analytical & laboratory services and discover the latest news from the contract services sector.
References
Upcoming Events
-
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025 -
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025 -
CPHI & PMEC China 2025
Shanghai New International Expo Center
24 Jun 2025 - 26 Jun 2025
Pharmaceutical Industry Webinars
-
Webinar Pathway to $10/g Biologics Production: Reshaping the Biomanufacturing Landscape
-
18th February 2025
-
3pm BST /4pm CET
-
-
Webinar An Untapped Market in Gender Inclusivity and Equity
-
19th March 2025
-
3pm GMT /4pm CET
-
-
Webinar Leveraging Real-Time Clinical Data to Deliver Certainty in Solubility Enhancement and Modified Release Development
-
12th December 2024
-
3pm BST/ 4pm CET
-
-
Webinar The CPHI Sustainability Collective: A New Initiative to Support a Sustainable Pharma Value Chain
-
10th December 2024
-
3pm BST /4pm CET
-
-
Webinar Key to Success: A CDMO's Pathway to Biologics Excellence
-
5th November 2024
-
3pm BST/ 4pm CET
-
-
Webinar The Changing Dynamics of Global API Manufacturing
-
17th September 2024
-
3pm BST/ 4pm CET
-
-
Webinar Shaping the Future of Italy’s Pharma Market: Trends and Opportunities
-
4th September 2024
-
4pm CET/ 10am EST
-
-
Webinar Fragment-Based Oligonucleotide and Oligopeptide Synthesis
-
30th Jul 2023
-
4pm CET / 10am EST
-
-
Webinar GMP Rationale for Sterile High-Potency/Toxic Pharmaceuticals
-
18th June 2024
-
4pm CET / 10am EST
-
-
Webinar Unlocking Opportunities in the Growing Pharma Landscape of The Middle East
-
5th June 2024
-
3pm CET / 9am EST
-
-
Webinar Exploring Technological Trends in the Future of Pharmaceutical Manufacturing
-
23rd May 2024
-
4pm CET / 10am EST
-
-
Webinar Achieving Manufacturing Excellence Through Digital Transformation
-
16th April 2024
-
4pm CET / 10am EST
-
-
Webinar Made in Africa: What’s Driving Pharma Manufacturing
-
28th March 2024
-
4pm CET / 10am EST
-
-
Webinar Case Study: Risk Management for Annex 1 Sterile Production EMS
-
28th February 2024
-
4pm CET / 10am EST
-
-
Webinar Innovative Strategies for B2B Pharma Marketeers: Driving Value through Content
-
20th February 2024
-
4pm CET / 10am EST
-
-
Webinar Revolutionizing Pharma: Data and AI Unleashed
-
18th January 2024
-
4pm CET / 10am EST
-
-
Webinar Optimal Temperature: Elevating Biologics Cold Chain Excellence
-
16th January 2024
-
4pm CET / 10am EST
-
-
Webinar Market Outlook – The Biggest Pharma Trends of 2024
-
12th December 2023
-
4pm CET / 10am EST
-
Recommended Products And News
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product QACS Pharmaceutical services
QACS Lab is GMP/GLP certified and provides contract laboratory testing for Pharmaceuticals. Methodologies stay in accordance with EMA & FDA. QACS is equipped with Microbiological - Chemical - Molecular - Packaging laboratory premises to provide variety of Pharmaceutical testing solutions &...
-
Product Drug Substance Services
CARBOGEN AMCIS provides comprehensive Drug Substance services, offering expertise in process development, scale-up, and manufacturing of active pharmaceutical ingredients (APIs). With state-of-the-art facilities, the company specializes in high-potency compounds, including cytotoxics, and delivers solu...
-
Product Analytical services
Our expertise spans across several critical areas:
Microbiology analysis • Microbial Identification (Micro ID) • Bioburden Testing • Endotoxin Testing Utilities Testing (Microbiology and Chemistry) • Water System Testing • Clean Steam Testing • Air Quality Monitoring Raw Ma...
-
Product Analytical package
XEDEV provides a broad range of analytical tools including : • Solid state analysis: mDSC, XRPD, PLM, TGA, FTIR, RAMAN • Powder characterisation : SEM, PSD (both wet and dry), flowability • Tablet characterisation : friability, disintegration, hardness, dissolution • Residual solvent determination :...
-
Product Trace Metals Testing and Elemental Analysis
Our experienced analysts apply a strategic approach to sample preparation for difficult samples and a range of analytical technology, relevant to your needs, including inductively coupled plasma – mass spectrometry (ICP-MS) or inductively coupled plasma – optical emission spectroscopy (ICP-OES) or ion chro...
-
Product Microbiology
Excerpt from the range of services offered by our microbiological department: • microbial contamination testing (bioburden) • identification of microorganisms • sterility testing • biological indicator testing • bacterial endotoxin testing (LAL), rFC testing
• assay of antibiotics • effi...
-
Product Ghost-Buster
Welch Ghost-Buster column can efficiently adsorb and remove the impurities from the mobile phase to eliminate their interference to the target peaks.
-
Product API Services, Chemical Development & Manufacture
Almac's strength in API development and manufacture is proven by being the partner of choice for many pharma and biotech companies seeking integrated drug development solutions from molecule to market.
Our technical expertise and extensive facilities enable us to offer integrated API contract ma...
-
Product Biotherapeutics
To accelerate the development and marketing of your Biotherapeutics, Quality Assistance offers a complete analytical package to meet the EMA and FDA requirements, all on one site.
Whether it is to extend your analytical capacities or to outsource parts or all of your analytical needs, ...
-
Product Formulation Development
Recipharm offer formulation development services for all dosage forms. We develop everything from simple formulations for early studies to more complex formulations suited for commercialisation.
-
Product Pharmaceutical Impurity Analysis and Identification Testing
Our GMP compliant laboratories provide pharmaceutical impurity testing for new drug substances (ICH Q3A(R2)) and new drug products (ICH Q3B(R2)) that can support your product development from an early stage and across the lifecycle of your drug product. Our scientists are adept at method routinely overcome...
-
Product Method Development, Validation and Transfer
Kymos Pharma Services, S.L. offers wide range of chemistry, manufacturing & control analytical services in medicinal chemistry which includes method development, validation and transfer. Kymos has comprehensive knowledge in the development and validation of analytical proprietary and non-proprietary method...
-
Product Quality control: Pharmaceuticals
Conscio offers GMP-certified and ICH-compliant chemistry, manufacturing and controls assays (CMC) as well as analytical support during validation of manufacturing processes. Samples are analyzed by approved and validated methods for routine batch testing, stability studies, analyses of high potency product...
-
Product Biocompatibility & Toxicology Testing
Nelson Labs offers a full trifecta of services to meet the requirements of ISO 10993 for Extractables & Leachables, Biocompatibility and Toxicological Assessments of medical devices. Biocompatibility testing is very common in the medical device industry. However, with 24 possible categories, each with...
-
Product Stability tests according to ICH
Neotron Pharma provides Stability tests according to ICH standards (zones I, II, IV). We can carry out stability studies in their entirety (storage + analysis) or provide only storage service. The conditions we can offer are listed below:
- 40°C ± 2°C , 75% ± 5% U.R.
- 30°C ± 2°C&n...
-
Product Analytical Services
Pharmaffiliates is providing all the Analytical Services (i.e. Method Development, Method Validation & Transfer, Stability Studies, Contract Analysis, etc)
-
Product Analytical services - OEL: 50 ng/m3
• Control of pharma samples solid state : Identification and quantification of solid state, method development and validation, routine QC. • Analytical techniques available : XRPD, DSC, TGA, DVS, Laser PSD, FTIR, IDR. • All analyses are applicable to toxic samples down to : 50 n...
-
Product Analytical Development
Idifarma's analytical development services include the following activities:
- Solubility studies in different media (organic, aqueous and different PH levels).
- Characterization of the thermal profile and identification of polymorphic forms by DSC.
- Impurities profile characterization. q...
-
Product Laboratory Services
Laboratory Services
Our 30 years of experience and our continual quest for improvement ensure we deliver analytical services of the highest level for active ingredients, excipients, medicinal products and drug-related products. In addition to developing and validating analytical methods, batch-...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance


















































































































































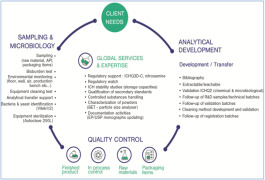
.jpg)

.jpg)






















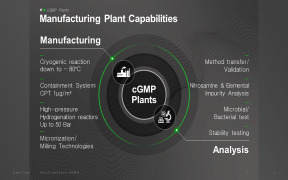
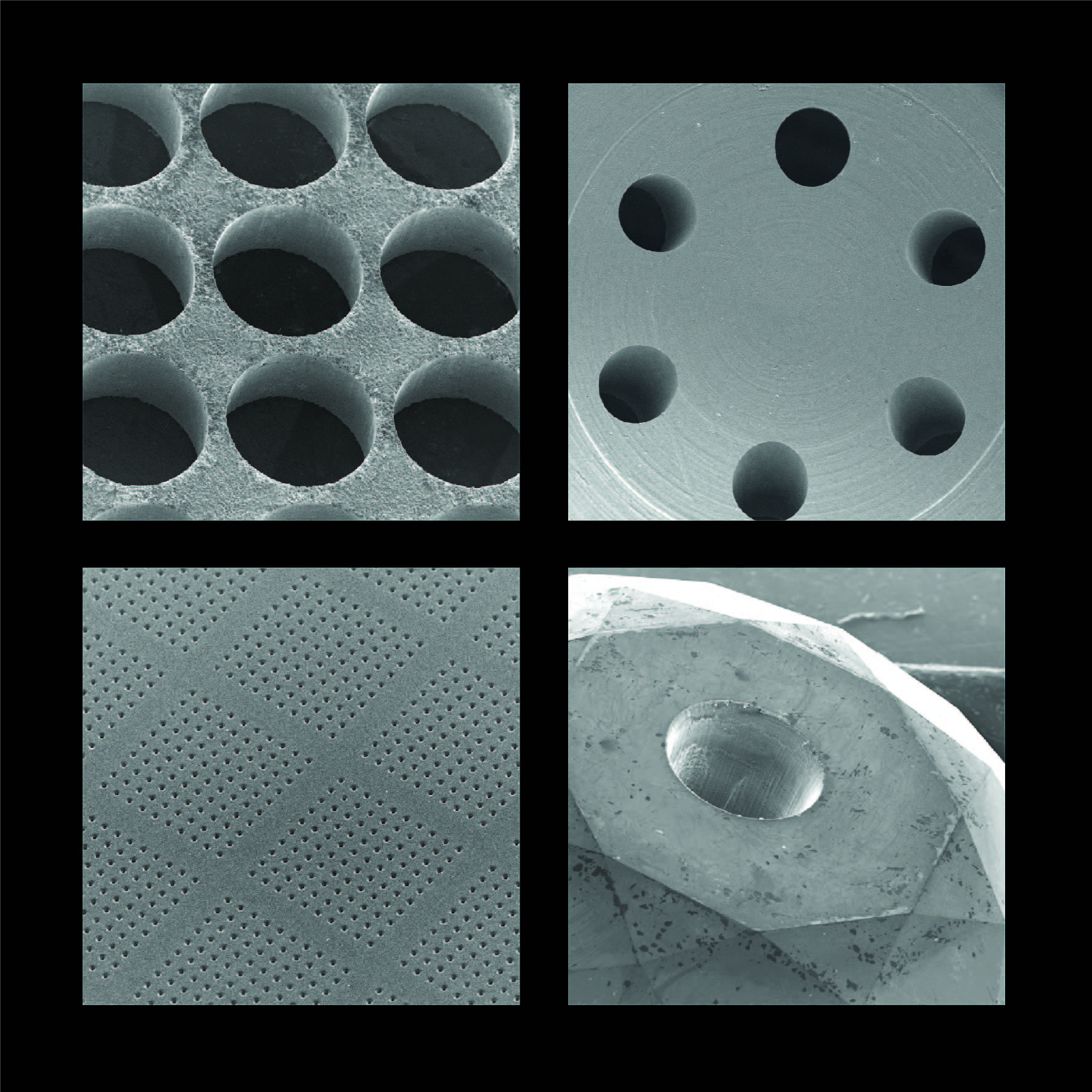























































.jpg)



















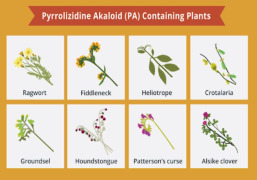




















.jpg)











.png)


.png)







.png)

.png)



.jpg)




















.jpg)

