Dossier Writing Service

Product Description
PHARMA-DATA S.A.

-
GR
-
2016On CPHI since
-
3Certificates
-
25 - 49Employees
Company types
Primary activities
Categories
Specifications
PHARMA-DATA S.A.

-
GR
-
2016On CPHI since
-
3Certificates
-
25 - 49Employees
Company types
Primary activities
More Products from PHARMA-DATA S.A. (18)
-
Product Stability Chambers
Pharma-Data sa offers wide range of products which includes stability chambers. Contact us for more information. -
Product Stability Testing Service
Pharma-Data sa offers wide range of services which includes stability testing service. According to ich guidelines covering zone iii and iv photo stability. Contact us for more information. -
Product Suppositories Filling Machine
Pharma-Data sa offers wide range of products which includes suppositories filling machine. Contact us for more information. -
Product Table Blister Machine / Mnf: O.M.A.R
Pharma-Data sa offers wide range of products which includes table blister machine / mnf: o.m.a.r. Contact us for more information. -
Product Analytical Development And Validation Services
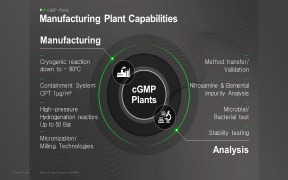
Pharma-Data sa offers wide range of services which includes analytical development and validation services. It includes justification of specifications, drug product release testing, method transfer, validation of microbiological methods (sterility, bet etc), filter validation (for sterile products – leach... -
Product Capsule Filling Machine
Pharma-Data sa offers wide range of products which includes capsule filling machine. Contact us for more information. -
Product Double-Jacketed, Vacuum, Heat/Cold Creams/Emulsion Mixer 5lt / Mnf: Stephan Gmbh – Germany
Pharma-Data sa offers wide range of products which includes double-jacketed, vacuum, heat/cold creams/emulsion mixer 5lt / mnf: stephan gmbh – germany. Contact us for more information. -
Product Fluid Bed Granulator 0.2-6kg / Mnf: Hüttlin Gmbh- Germany
Pharma-Data sa offers wide range of products which includes fluid bed granulator 0.2-6kg / mnf: hüttlin gmbh- germany. Contact us for more information. -
Product High Shear Batch Mixer-Granulator 5lt / Mnf: Hüttlin Gmbh – Germany
Pharma-Data sa offers wide range of products which includes high shear batch mixer-granulator 5lt / mnf: hüttlin gmbh – germany. Contact us for more information. -
Product Ipcs Equipment
Pharma-Data sa offers wide range of products which includes ipcs equipment. Contact us for more information. -
Product Junior E Xpress (8 Punches Rotary Tableting Machine) / Mnf: Talleres Sanchez Srl
Pharma-Data sa offers wide range of products which includes junior e xpress (8 punches rotary tableting machine) / mnf: talleres sanchez srl. Contact us for more information. -
Product Lab Scale Autoclave 25l (105-134oc) / Mnf: Grouppo-Selecta – Spain
Pharma-Data sa offers wide range of products which includes lab scale autoclave 25l (105-134oc) / mnf: grouppo-selecta – spain. Contact us for more information.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance










-comp246698.jpg)