Method Validation
Method Validation Companies (43)
Method Validation News
-
News Quality laboratory data with PPD Laboratories
Quality laboratory data is vital to your pharmaceutical development program. -
News High demand encourages Sterling Pharma's phased expansion of its US facility
The investment will allow the CDMO to take on new projects, as well as bolster the company's chemistry development capabilities. -
News Specialist API CDMO acquired by GHO Capital
Future plan is to extend Sterling’s international presence to meet increasing demand from customers worldwide.
Method Validation Products (48)
-
Product Inhalation Drug Product Development Services
Services for the development of inhalation drugs: Our team have over 30 years of experience supporting our clients' product development for orally inhaled or nasal drug products (OINDP). This includes formulation, stability, testing, product performance testing, in vitro bioequivalence studies, CMC...
-
Product Extractables & Leachables Testing
Nelson Labs Europe offers a comprehensive approach toward Extractables & Leachables testing for the pharmaceutical industry. Our approach combines technical and analytical expertise, polymer knowledge and understanding of regulatory requirements, all combined in a tailored approach for our customers.
-
Product Development
Synerlab provides wide range of services which includes development. Contact us for more information.
-
Product QACS Pharmaceutical services
QACS Lab is GMP/GLP certified and provides contract laboratory testing for Pharmaceuticals. Methodologies stay in accordance with EMA & FDA. QACS is equipped with Microbiological - Chemical - Molecular - Packaging laboratory premises to provide variety of Pharmaceutical testing solutions &...
-
Product Innovation & Formulation
Innovation & Formulation: We offer pharmaceutical R&D, feasibility studies, proof-of-concept, formulation development and analytical method development for multiple dosage forms such as solids, semi-solids, liquids, oral films, and transdermal patches. We can work with over 30 pharma technologies f...
-
Product Analytical Services
Pharmaffiliates is providing all the Analytical Services (i.e. Method Development, Method Validation & Transfer, Stability Studies, Contract Analysis, etc)
-
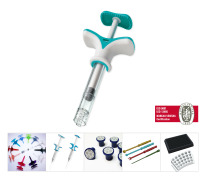
Product Medical Devices for various medical procedure (HA injection syringes accessories, Blood analysis, surgical tool, orthopedics...)
De l'idée à la conception, l'industrialisation jusqu'à la qualification et la production.
From the idea trough the design, industrialization until qualification and production.
-
Product Method Validation
Neotron Pharma is able to validate analytical methods developed internally or transmitted by the customer using top of the range equipment such as:
- HPLC-UV / DAD / MSMS
- GC-FID / ECD / MSMS (even in Headspace)
- ICP-MS / AAS / AES / OES
- Post-column derivatization
- Particle si...
-
Product Wasdell Laboratory Services
By the end of 2017 we will have a MHRA, GMP licenced laboratory. We will be offering a wide range of services for routine Quality Control analysis including QC release testing, method validation and stability analysis (analytical and microbiological). We work to the highest standards of GMP and GLP with r...
-
Product Sterility Assurance Testing
Nelson Labs is a leading provider of sterility assurance test services for medical device, pharmaceutical and tissue manufacturers for both sterile and nonsterile products. Most tests follow United States or European Pharmacopeia (USP or EP) and similar international standards. Medical device standards are...
-
Product Analytical Testing and Formulation Development
Quality Chemical Laboratories is known for its capabilities in the development and validation of analytical methods for raw materials, drug substances, and drug products. With a strong focus on quality and precision, QCL employs cutting-edge technologies and a highly skilled team of scientists to offer com...
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product Laboratory & Analytical Services
Avéma has a full analytical lab, staffed by experienced professionals with more than 200 years of method development and validation experience.
-
Product Laboratory Services
Curida offers Lab services1 chemical lab
3 microbiological labs, including sterility lab and lab for test organisms
Main services:
Quality Control
Method development and validation
Stability testing
Contract laboratory
-
Product Development and Validation of Analytical Methods
Analytical methods development and validation: Our method development scientists work with a broad range of products, methods, and analytical technologies (chromatography, mass spectrometry, spectroscopy, biophysical analysis, bioanalytical techniques, etc.) to ensure that the method will meet its int...
-
Product Facility & Process Validation Testing
Manufacturing facilities and processes can be a primary source of product contamination. Nelson Labs offers a range of services to assess these manufacturing environmental conditions, in addition to water system validations, raw material screening and residual manufacturing material tests.
-
Product Sterility Assurance Testing
Nelson Labs is a leading provider of sterility assurance test services for medical device, pharmaceutical and tissue manufacturers for both sterile and nonsterile products. Most tests follow United States or European Pharmacopeia (USP or EP) and similar international standards. Medical device standards are...
-
Product Packaging Solutions Testing
We offer unparalleled breadth of packaging experience to help our clients deliver safe and effective products to market. Our services encompass consulting, package validation, material qualification and package development. Nelson Labs packaging solutions department has experience with successful valida...
-
Product Biocompatibility & Toxicology Testing
Nelson Labs offers a full trifecta of services to meet the requirements of ISO 10993 for Extractables & Leachables, Biocompatibility and Toxicological Assessments of medical devices. Biocompatibility testing is very common in the medical device industry. However, with 24 possible categories, each with...
-
Product Pharmaceutical Impurity Analysis and Identification Testing
Our GMP compliant laboratories provide pharmaceutical impurity testing for new drug substances (ICH Q3A(R2)) and new drug products (ICH Q3B(R2)) that can support your product development from an early stage and across the lifecycle of your drug product. Our scientists are adept at method routinely overcome...
Upcoming Events
-
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025 -
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance












































.jpg)















































.png)







.png)

.png)



.jpg)


