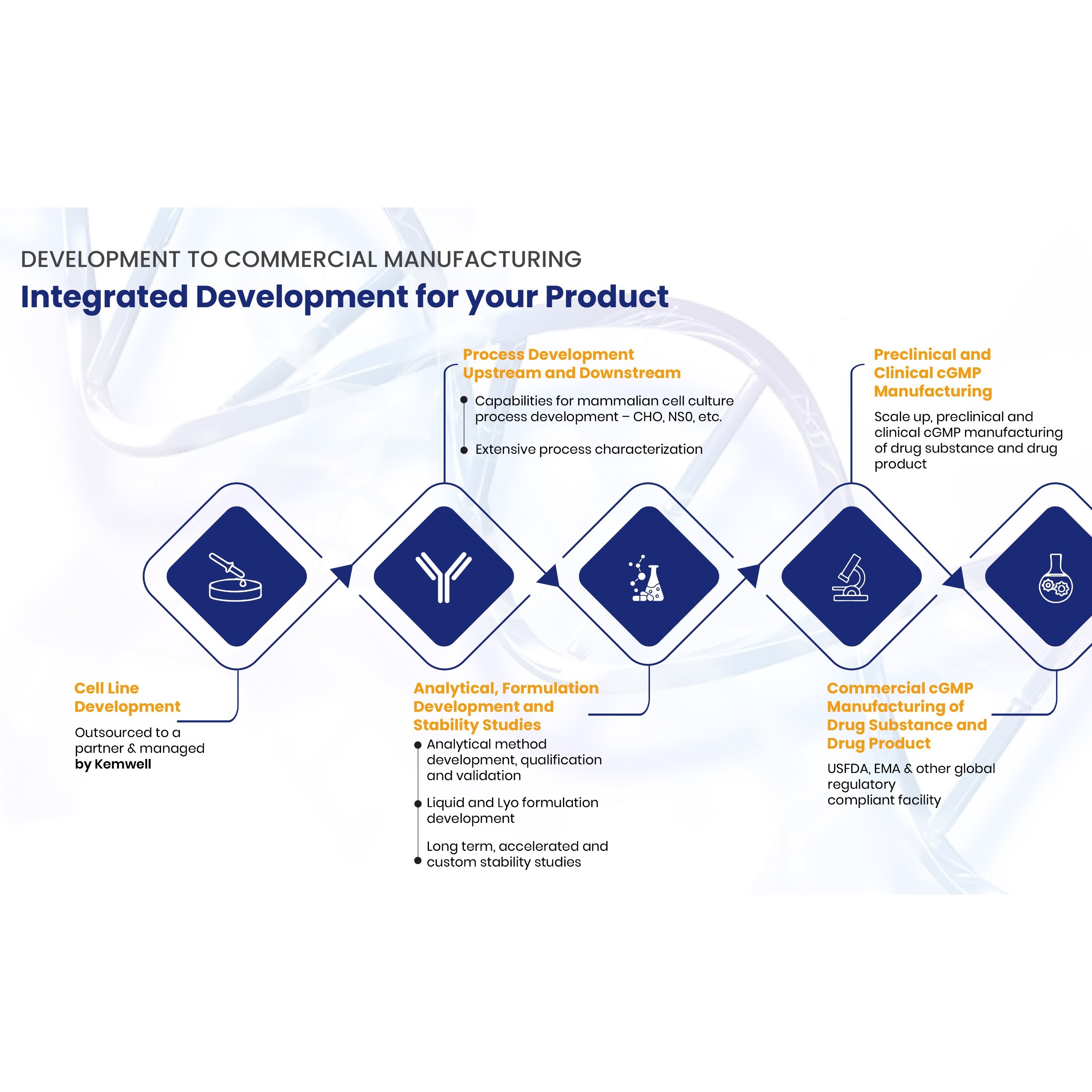
Analytical Services
Product Description
Pharmaffiliates Analytics & Synthetics Pvt. Ltd.

-
IN
-
2015On CPHI since
-
3Certificates
-
250 - 499Employees
Company types
Primary activities
Categories
Specifications
Pharmaffiliates Analytics & Synthetics Pvt. Ltd.

-
IN
-
2015On CPHI since
-
3Certificates
-
250 - 499Employees
Company types
Primary activities
More Products from Pharmaffiliates Analytics & Synthetics Pvt. Ltd. (99)
-
Product (?)-n-butylscopolamine bromide; (?)-scopolamine n-butyl bromide; hyoscine n-butyl bromide;
Pharmaffiliates offers a wide range of products which includes (?)-n-butylscopolamine bromide; (?)-scopolamine n-butyl bromide; hyoscine n-butyl bromide;. It belongs to hyoscine butylbromide category. Contact us for more information. -
Product (?)-norepinephrine; (r)-4-(2-amino-1-hydroxyethyl)-1,2-benzenediol; l-arterenol; l-noradrenaline; levarterenol
Pharmaffiliates offers a wide range of products which includes (?)-norepinephrine; (r)-4-(2-amino-1-hydroxyethyl)-1,2-benzenediol; l-arterenol; l-noradrenaline; levarterenol. It belongs to adrenaline tartrate category. Contact us for more information. -
Product (?e)-?-[[2-butyl-1-[(4-carboxyphenyl)methyl]-1h-imidazol-5-yl]methylene]-2-thiophenepropanoic acid
Pharmaffiliates offers a wide range of products which includes (?e)-?-[[2-butyl-1-[(4-carboxyphenyl)methyl]-1h-imidazol-5-yl]methylene]-2-thiophenepropanoic acid. It belongs to eprosartan category. Contact us for more information. -
Product (?e)-?-[[2-butyl-1-[[4-(methoxycarbonyl)phenyl]methyl]-1h-imidazol-5-yl] methylene]-2-thiophenepropanoic acid ethyl ester
Pharmaffiliates offers a wide range of products which includes (?e)-?-[[2-butyl-1-[[4-(methoxycarbonyl)phenyl]methyl]-1h-imidazol-5-yl] methylene]-2-thiophenepropanoic acid ethyl ester. It belongs to eprosartan category. Contact us for more information. -
Product (?r,?r)-6-amino-?,?-dihydroxy-9h-purine-9-butanoic acid
Pharmaffiliates offers a wide range of products which includes (?r,?r)-6-amino-?,?-dihydroxy-9h-purine-9-butanoic acid. It belongs to adenosine category. Contact us for more information. -
Product Reference Standards
Pharmaffiliates is Supplying all the Pharmacopoeial Reference Standards (i.e. USP, EP, BP, IP, JP).Apart from this we offer Pesticide Standards, Phyto-chemical Standards, Food & Environmental Standards, etc.
For more details please send your enquiry on marketing@pharmaffiliates.com -
Product Impurity Synthesis
Pharmaffiliates Analytics & Synthetics (P) Ltd, is an integrated CRO (Contract Reserach Organisition) established in year 2001. Presently our group consists of more than 130 Scientists with 3 R&D Centres offering its expertise in Custom Synthesis, Impurity synthesis, Isotoped lebelled compound... -
Product Regulatory Services
Pharmaffiliates providing the full support for the Regulatory Services (i.e. DMF Filling, Dossier Preparations, etc) -
Product Stability Studies
Pharmaffiliates offers Stability Studies. -
Product Formulation & Development
Formulation Product development team includes experienced Scientists who continuously work for the development of New Drug Product, Dietary Supplement, Generic Drug Products, Novel Drugs Delivery System (NDDS), New fixed dose combination products and Herbal Cosmetics & Herbal Products.
We offer... -
Product CRAMS
"A reliable and preferred CRAMS (Contract Research And Manufacturing Services) partner in providing innovative Pharmaceutical solutions"
SERVICES OFFERED:* Early phase drug development* Process research and development* CCS (Custom chemical synthesis)* FTE (Full Time Equivalent) based projects&...
Pharmaffiliates Analytics & Synthetics Pvt. Ltd. resources (1)
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






















.jpg)