Analytical Services
Product Description
QpLab - Pharma Services, Lda

-
PT
-
2019On CPHI since
-
2Certificates
-
1 - 24Employees
Company types
Categories
Specifications
QpLab - Pharma Services, Lda

-
PT
-
2019On CPHI since
-
2Certificates
-
1 - 24Employees
Company types
More Products from QpLab - Pharma Services, Lda (3)
-
Product Batch Testing & EU Batch Release / Certification
QP Declaration
QPLab will evaluate the information regarding active substances manufacturing sites of the product, to assess the EU GMP compliance and issuing the QP Declaration.
QP Batch Certification SetupIn this step QPLab will have a complete oversight ... -
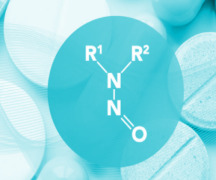
Product Nitrosamines - QC / Development / Validation
LC-MS/MS methods are nowadays commonly used for nitrosamines analysis in all types of materials, since it allows analyte-specific detection based on both retention time and structurally specific fragmentation information in conjunction with high sensitivity.
After the establishment of the ni... -
Product Medicinal Cannabis
QPLAB has GMP Cannabis Testing licencing that allow to provide the following services: • Medicinal Cannabis Testing • Batch Testing • Enviromental Monitoring • Stability Studies • Method Development • Research Formulations Testing capacity:
• Visual Examination • Loss on drying • Deter...
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance