Stability Studies
Product Description
QACS, The Challenge Test Lab

-
GR
-
2016On CPHI since
-
4Certificates
-
100 - 249Employees
Company types
Primary activities
Categories
QACS, The Challenge Test Lab

-
GR
-
2016On CPHI since
-
4Certificates
-
100 - 249Employees
Company types
Primary activities
More Products from QACS, The Challenge Test Lab (5)
-
Product Molecular Testing solutions
Our Lab uses molecular technology to provide pharmaceutical testing services in rapid turnaround times. DNA Microlab, member of QACS assists pharmaceutical manufacturing and monitoring processes in order to secure the absence of microbial contamination via Microbial Species Identification, Pathogen detecti... -
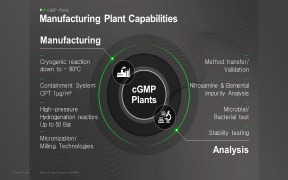
Product QACS Pharmaceutical services
QACS Lab is GMP/GLP certified and provides contract laboratory testing for Pharmaceuticals. Methodologies stay in accordance with EMA & FDA. QACS is equipped with Microbiological - Chemical - Molecular - Packaging laboratory premises to provide variety of Pharmaceutical testing solutions &... -
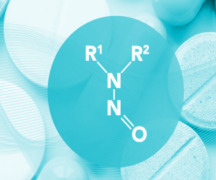
Product Nitrosamine Detection
We provide Method Development and Method Validation for new analytical methods or Method Transfer for Routine Testing according to existing validated methods for raw materials and final products. QACS is GMP/GLP certified.
• performed with UPL... -
Product Leachables Extractables
QACS tests for organic and inorganic components, Volatile, Nonvolatile residues and perform Elemental impurities analysis. Packaging Migration/Compatibility studies are also provided. Studies are performed with state of the art equipment.
▶️ info@qacslab.com +30 21... -
Product Method Validation & Transfer
Pharmaceutical testing services during and after drug development from QACS ensure GMP compliance and product safety. Method validation studies aim to demonstrate analytical suitability of procedures according to their intended use/purpose. QACS transfer method protocols ensure, that results generated by s...
QACS, The Challenge Test Lab resources (5)
-
News US FDA Registration Certificate
QACS Ltd Is registered with the US FDA as an analysis laboratory for the statutory filing period applicable to US FY 2021 pursuant to part 207 of Title 21 US Code of federal Regulations -
Brochure QACS_Laboratory Testing Services
Check our brochure! All of our team members will be happy to assist you further with any queries you might have! Contact info@qacslab.com - (+30) 210 29 34745Visit www.qacslab.com -
Brochure QACS Lab has acquired Crédit d’Impôt Recherche (CIR) from the French Ministry of Higher Education, Research and Innovation for the years 2021-2023 The research tax credit is a system that supports research and development activities for companies that ar
QACS Lab has acquired Crédit d’Impôt Recherche (CIR) from the French Ministry of Higher Education, Research and Innovation for the years 2021-2023
The research tax credit is a system that supports research and development activities for companies that are subject to corporate tax in France.
Aiming to encourage organizations to develop their research and development activities by providing them with a tax deduction.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance











































































-comp296622.jpg)



-comp246698.jpg)