Solid & Liquid Dose Drug Manufacturing & Development

Product Description
Avema Contract Servies

-
US
-
2017On CPHI since
-
1Certificates
Company types
Categories
Avema Contract Servies

-
US
-
2017On CPHI since
-
1Certificates
Company types
More Products from Avema Contract Servies (9)
-
Product Formulation Development
Avéma has an extensive library of formulations and more than 40 years of Rx formulation development experience. -
Product Gummy Manufacturing
Avéma Pharma Solutions and PL Developments has responded to the increasing demand for gummies by adding gummy manufacturing capabilities, including a small line for R&D and scale up, as well as four additional commercial lines with an anticipated capacity of 1.2bn gummies annually. Commercially available p... -
Product Laboratory & Analytical Services
Avéma has a full analytical lab, staffed by experienced professionals with more than 200 years of method development and validation experience. -
Product Packaging & Serialization
Avéma can help you improve patient compliance with innovative packaging solutions for solid and liquid dose productions. Our facilities offer flexibility to accommodate run size and interchangeable tooling for line flexibility and cost efficiency – so, we can provide support for all products at all stages ... -
Product Pilot Scale Manufacturiing
To ensure the seamless transition from pilot manufacturing, Avéma starts with small-scale production equipment in the development process that has identical operational attributes to what will be used in full-scale commercial manufacturing all under the supervision of a single project manager to ensure tha... -
Product Regulatory Support
Avéma Pharma Solutions can help guide your product successfully through NDA, ANDA, CBE 30 and 505(b)(2) filings with the FDA. -
Product Research & Development
Avéma offers formulation, process and analytical development support of new or existing products using the most current and effective active ingredients and delivery systems. Our team of scientists bring decades of pre-formulation development expertise to your products, combined with the knowledge of how t... -
Product ANDA and NDA Technical Support
As part of its commitment to full service support, Avéma Contract Services is pleased to offer our customers support with ANDA and NDA applications, including bio equivalency studies, collecting background data, supervising technology tranfers and reviewing applications to make sure that companies com... -
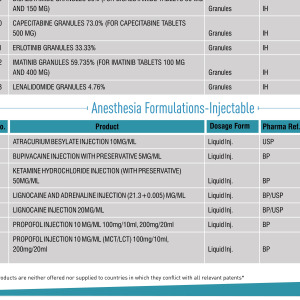
Product Commercial Manufacturing
Covering a 135,000ft² area, our Miami facility is registered with the FDA and complies fully with current good manufacturing practice (cGMP) guidelines. It has the capacity for R&D, process development and small-scale launches, including pilot-scale cGMP manufacturing and packaging to support pilot pharmac...
Avema Contract Servies resources (12)
-
Brochure Lessons Learned: Choosing & Managing a CDMO
Lessons Learned: Choosing & Managing a CDMOAvéma Pharma Solutions, a division of PL Developments (PLD), is in the unique position being both a consumer and supplier of global CDMO services for both OTC and rX products. In this presentation, we’ll explore the lessons we have learned from managing our own CMOs that have allowed us to build a better CDMO model for our Avéma customers. We’ll address the areas that can help speed up your time-to-market, keep development costs under control, and smooth the FDA approval and commercial launch process. Watch our webinar at https://app.webinar.net/K2OlZE6JLx9?mcc=WebsitePost
-
Video Welcome to Avema Contract Services
Avéma Contract Services is one of the only Contract Development and Manufacturing Organization (CDMO) that offers integrated, turnkey, end-to-end solutions for Rx, OTC and nutraceutical liquid and solid dose delivery systems. Headquartered in Westbury, NY, Avéma has manufacturing and distribution locations in Miami, Fla., Lynwood, Ca., and Greenville, S.C. Avéma Contract Services is powered by PL Developments. -
Video Overcoming the Challenges of Gummies as a Drug Delivery System
The global gummy vitamin market is expected to reach 10.6 billion by 2025 and gummies are gaining traction as a drug delivery system -- especially for children and seniors who resist taking pills, capsules and liquids. Mitchell Slade, who brings more than 20 years of gummy manufacturing experience at Nature’s Bounty to the table, will address the challenges of developing gummies beyond supplements, such ensuring consistent dosage, production and taste. Avéma/PLD has a world-class GDUFA facility that is CFR 211 and CFR 111 certified, uses a starchless gummy manufacturing process and brings 40+ years of formulating experience to the development of innovative drug delivery systems. -
Video The Promise of Gummies as a Drug Delivery System
The global gummy vitamin market is expected to reach 10.6 billion by 2025 and gummies are gaining traction as a drug delivery system -- especially for children and seniors who resist taking pills, capsules and liquids. Mitchell Slade, who brings more than 20 years of gummy manufacturing experience at Nature’s Bounty to the table, will address the challenges of developing gummies beyond supplements, such ensuring consistent dosage, production and taste. Avéma/PLD has a world-class GDUFA facility that is CFR 211 and CFR 111 certified, uses a starchless gummy manufacturing process and brings 40+ years of formulating experience to the development of innovative drug delivery systems. -
Brochure The Promise of Gummies as a Drug Delivery System
Avéma is leveraging its OTC and Rx drug development expertise into using gummies as a novel delivery system. This White Paper describes what it takes to succeed in this expanding market. -
Brochure Avéma Pharma Solutions: Capabilities
This presentation provides an overview of Avéma Pharma Solution's capabilities. -
Whitepaper The Promise of Gummies as a Drug Delivery System
Looking at the supplement industry as a barometer for drug delivery systems, it’s apparent that gummies are gaining traction as a preferred delivery system. In 2019, the sales of non-pill formats, including gummies, powders and shots, exceeded the sales of pill formats and that gap between the pill and non-pill market share continues to grow. -
Brochure Avéma Pharma Solutions Building New R&D Center
Avéma Pharma Solutions is building an enhanced, state-of-the-art R&D center that is almost three times larger than the existing space and will help the company respond to increased customer demand for formulation and analytical development. -
Brochure New Year, New Capabilities for Avéma Pharma Solutions
Avéma Pharma Solutions has made significant investments to enable new capabilities and expand its service offerings going into 2023. -
Brochure Avéma Pharma Solutions Manufacturing and R&D
This presentation touches on how Avéma Pharma Solutions helps to eliminate lost time while transitioning from R&D to commercial launch.
-
Video Take a tour of Avéma's R&D facilities.
Aaron Dely, Avéma's Senior Director of Global Research, gives a tour of Avéma's R&D facilities and discusses how the company's focus on small batches with short turn times around allows for more characterization of the process through design of experiments to support the FDA’s mandate on quality by design. This approach can lead to reductions in review time by the FDA and lead to fewer information requests or a faster approval cycle.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

















-file148142.png)































































-comp273426.jpg)