Regulatory Testing and Research based services for Pharmaceuticals, Chemicals, Phytochemicals, Herbal Formulations, Food and Medical devices
Product Description
Accuprec Research Labs Pvt Ltd

-
IN
-
2019On CPHI since
-
1Certificates
-
100 - 249Employees
Company types
Primary activities
Categories
Specifications
Accuprec Research Labs Pvt Ltd

-
IN
-
2019On CPHI since
-
1Certificates
-
100 - 249Employees
Company types
Primary activities
More Products from Accuprec Research Labs Pvt Ltd (4)
-
Product Medical Device Testing
1) Physico-Chemical Testing of Raw Material & Finished Medical Devices2) Biocompatibility Testing of Medical Devices(As per ISO 10993-1:2018)
3) Biological Testing of Raw Material of Plastic, Rubber,Silicone, Polymers, etc.(As per IP / BP / EP / JP / USP)
4) Microbiological Testing Services ... -
Product Complete Characterization Testing Solution For Iron Carbohydrate
Method Development & Validation @ACCUPREC
1) Iron Chelation Assay (labile iron by U.V spectroscopy) Validated method
2) Polymorphic estimation of Iron carbohydrate by XRD
3) Estimation ratio of Fe3+ to Fe2+ ion validated method
4) Morphology estimation of iron carbohydrate
5)... -
Product Analytical Testing Of Pharmaceuticals & Cosmetics
• Analytical Testing of Pharmaceuticals & Cosmetics • API characterization and analysis Analytical method development • Bio-Analytical Method Development and Validation • Comparative Dissolution Study • Excipient compatibility Study • Extractable and Leachable Study (As per USP) • Impurity Isolation and Ch...
Accuprec Research Labs Pvt Ltd resources (3)
-
Brochure Corporate Brochure
At Accuprec, we offers below mentioned various services :
1) Analytical Testing of Pharmaceuticals & Cosmetics
2) Biotechnological Services
3) Microbiological Services
4) Bio - Compatibility Studies of Medical Devices (As per ISO 10993, USFDA and EU MDR Guidelines)
5) Preclinical & Toxicological Services (As per OECD, NDCT, ICH, MHRA, USFDA/EPA Guidelines)
6) Phytochemical & AYUSH Testing Services (As per AYUSH and other Guideline)
7) Food Testing Services (As per FSSAI, BIS, APEDA and EU Guideline)
8) Formulation & Development Servies
9) Dyes, Pigment & Plastic Testing Services
10) Research & Development Services (DSIR approved R & D centre)11) Stability Testing Services
12) Clinical Services
13) Regulatory Dossier Preparation
-
Brochure Medical Device Testing Brochure
1) Physico-Chemical Testing of Raw Material & Finished Medical Devices2) Biocompatibility Testing of Medical Devices (As per ISO 10993-1:2018)
3) Biological Testing of Raw Material of Plastic, Rubber, Silicone, Polymers, etc.(As per IP / BP / EP / JP / USP)
4) Microbiological Testing Services
5) Packaging Testing
6) Stability Testing Services
7) Mask, PPE, Gloves & Textile Testing
8) Performance Testing of Medical Devices
9) Performance Testing of Rapid In-Vitro Diagnostic Kits
10) Research & Development Services
11) Clinical Study
12) Regulatory Dossier Preparation Services
13) IPR Management Services
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance
























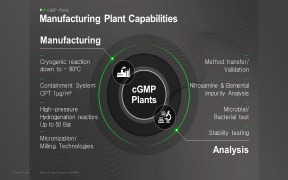






-comp246698.jpg)