- Home
- MYER RESEARCH Inc.
- Preclinical Laboratory Services
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
Preclinical Laboratory Services
Product Description
MYER's team counts with 30+ years of experience conducting nonclinical efficacy in vitro and in vivo experiments. Small molecules, biologics, devices, diagnostics. Contact us to get a quote!
MYER RESEARCH Inc.
-03-comp324100.jpg)
-
US
-
2024On CPHI since
-
1 - 24Employees
Company types
Consultancy
Contract Research Organisation (CRO)
Contract Service
Pharmaceutical company
Start-up
Primary activities
Clinical Research
Contract Research Organisation
Laboratory Services
Sales Markets
MYER RESEARCH Inc.
-03-comp324100.jpg)
-
US
-
2024On CPHI since
-
1 - 24Employees
Company types
Consultancy
Contract Research Organisation (CRO)
Contract Service
Pharmaceutical company
Start-up
Primary activities
Clinical Research
Contract Research Organisation
Laboratory Services
More Products from MYER RESEARCH Inc. (1)
-
Product Nearshore Clinical Trial - Broker Service
MYER counts with 5+ years of experience brokering clinical trials for US-based companies in Mexico. If you want to reach diverse populations and epidemiology, enter a new market, and/or save up to 50 % of the cost compared to a US trial, then contact us to get a quote!
Recommended Products
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product cGMP Manufacturing
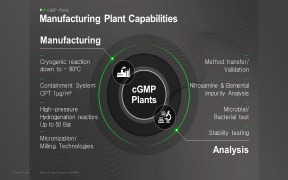
1. Manufacturing facilities ensure: 1) Production flexibility at various scale 2) Total Production Capacity: 726 m3 including pilot production 3) Potent compound manufacturing (OEL Category 3A, CPT 1 Mug/m3) 2. Various Analytical Capabilities: 1) cGMP QC lab with HPLC, UPLC, GC, LC-MS, GC-MS, NMR, X...
-
Product Analytical Services
Physico-Chemical testing
- General identification (TLC, HPLC)
- Assay (UV/VIS, HPLC-UV, GC-FID, LC-MS/MS) and dosage uniformity
- Related substances identification and quantification (HPLC-UV, GC-FID, LC-MS/MS)
- Physical determinations (pH, viscosity, density)
- Moisture...
-
Product Impurity Synthesis
Pharmaffiliates Analytics & Synthetics (P) Ltd, is an integrated CRO (Contract Reserach Organisition) established in year 2001. Presently our group consists of more than 130 Scientists with 3 R&D Centres offering its expertise in Custom Synthesis, Impurity synthesis, Isotoped lebelled compound...
-
Product Solid & Liquid Dose Drug Manufacturing & Development
From OTC and Rx to diagnostics and dietary supplements, Avéma manufactures a full range of solid and liquid dose products, all manufactured under strict FDA guidelines and cGMP compliance. With an ever-growing portfolio of innovative formulas and a diverse mix of state-of-the-art equipment, our offerings a...
-
Product Netpharmalab Consulting Services
Contract Research Laboratory. Analytical Services (physicochemical analysis, microbiological analysis and stability studies) and EU Import Lab.
-
Product SOFTGELS
At Softigel, we offer much more than traditional softgels, including everything from a single unit dosage form, to multiple delivery systems in a single dose. Softgels are an effective delivery system for oral drugs, especially those with low solubility and/or permeability (BCS Classes II, III and...
-
Product Analytical Services
From a drug product’s first raw materials to its release-ready batches, our experts rigorously evaluate and verify a product’s quality at key steps throughout the manufacturing process. Analytical services include material testing to verify product quality from the early stages of development, cGMP-com...
-
Product Stability Studies
QACS Labs offer drug development services. Pharmaceutical testing includes pharmaceutical stability testing, storage stability services and custom solutions based on international standards. We test API’s, raw materials and final products in recommended environmental storage conditions such as Temperature,...
-
Product Disease Activity Models/Tissue Culture
MedPharm has developed proprietary models relevant for major disease areas such as psoriasis, atopic dermatitis, onychomycosis and continue to develop new ones through our dedicated innovation group. These sophisticated ex vivo experiments provide additional confidence to clients and their in...
-
Product Fermentation
Relying on an experience gained over more than 50 years, OLON represent one of the most extensive know how of microbial fermentation in Europe. The Group, global leader in biomanufacturing, has two Biotechnology Centres located in Italy and is one of the first companies in Italy producing via microbial ...-comp246698.jpg)
-
Product Biologics Characterization
SGS’ range of services dedicated to biopharmaceutical product characterization bring the most recent developments in testing technology to companies across the globe. In 2010, SGS acquired the M-Scan Group, the world leaders in the application of advanced mass spectrometry
techniques for protein and c...
-
Product Syringe and Vial Filling for Clinical and Commerical
Lifecore’s experts have gained the know-how to build processes around your requirements for smooth scale-up and high-quality manufacturing.
Whether we’re your primary partner or a secondary source for manufacturing, we ensure you're fully satisfied with the final produ...
-
Product Wasdell Manufacturing
Wasdell’s specialised pharmaceutical manufacturing facilities in the North East and the East Midlands are MHRA approved for the manufacture of non-sterile oral liquids with the capability and capacity to extend its services to other dosage forms. We can support the manufacture and packaging for cl...
-
Product R&D Services
Our R&D Services are categorized into 8 streams: • Material Science Services
• Metal and Organic Scavenging Screening Services
• Organic Synthesis Services
• Catalysis Services
• Process Services
• Chromatography and Purification Services
• Method D...
-
Product Development of Formulation
Formulation Development- Cosmetic products
- Medical devices (skin and mucous membranes)
- Oral supplements
- Veterinary products
- Biocidal products
- Topical medicines
Stability tests - evaluation of the stability of cosmetic products, medical devices, food supplements and bioci...
-
Product Lyophilization Process Development (Quality By Design approach)
With the aim of obtaining a product that meets the critical quality attributes, but with the most optimized freeze-drying process.
Our focus is the total control of the process. We develop freeze-drying processes in a previously defined design space: Quality By Design approach.
-
Product VialArch
The Gasporox sensor module VialArch offers non-destructive Headspace Gas Analysis to be integrated directly onto the production line or into an inspection machine.
This unique laser-based solution is installed on the production line for 100% quality inspection and container closure ...
-
Product Regulatory Testing and Research based services for Pharmaceuticals, Chem...
1) Analytical Testing Of Pharmaceuticals & Cosmetics2) Biotechnological Services
3) Microbiological Services
4) Bio - Compatibility Studies of Medical Devices(As per ISO 10993USFDA and MHLW Guideline)
5) Preclinical & Toxicological Services
6) Phytochemical & AYUSH Testing Se...
-
Product Research & Development Capabilities
PHT International Inc. offers a wide range of services which includes research & development capabilities. Features: it includes process development, process improvement, sample repackaging, sample production, sample analysis. Contact us for more information.
-
Product J.T.Baker® Direct Dispense packaging system
Enhance the effectiveness of your products and streamline your biopharmaceutical manufacturing processes with the J.T.Baker® Direct Dispense packaging system. This novel packaging platform makes it easy to deliver powdered performance materials and excipients directly to your process, whether you are using...
-
Product Bioanalysis of Oligonucleotides
We quantify according to GLP nucleic acids in various biological matrices for you. We develop standardized and reproducible methods that will bring you through FDA/EMA registration. No matter which nucleic acids you develop as therapeutics, biomarkers or similar, we offer you the quantification of your nuc...
-
Product Inhalation Drug Product Development Services
Our pharmaceutical auditing and management services give you a transparent view of your supply chain enabling you to identify and mitigate the intrinsic risks in your operations, supply chains and business processes. Through our shared audit programs, delivered by our global network of specialist ...
-
Product Microbiology
Microbiological testing is an important factor in ensuring your product’s safety, efficacy, and timely project completion. At Alcami, we combine decades of microbiological expertise, developed from serving the biotech, pharmaceutical, and medical device industries, to provide the most current and effective...
-
Product Analytical Development and expertise
Our analytical experienced team support the pharmaceutical development during every phase of the development process. Analytical development transfer and validation, characterization capabilities, stability analysis, ICH Q3D (residuals solvent risk assesment), ICHQ3C (elemental impurities risk assesment...
-
Product Analytical Services
With 55+ years of experience, our expert teams develop 1,000+ analytical methods and validate 250+ methods annually. Drawing upon an extensive range of analytical technology, combined with a wealth of analytical knowledge, we can add real value to your drug development and commercialisation programs....
-
Product Core Technologies and Services
• API / GMP Manufacturing • Rapid Process Development, Flawless Upscaling, and Economy of Scale-Production • Simulated-Moving Bed (SMB) Chromatography • Heterocyclic, Hazardous and Malodorous Chemistries • Organometallic and Cryogenic Chemistry • Transition-Metal Catalysis • High-Pressur...
-
Product Analytical Services
ChemCon’s analytics team takes care of your inquiries if you are looking for ICH-compliant quality control, GMP validation, release analysis, impurity determination, reference standards or answers to other analytical queries. Close, outcome-oriented communication is the key to a successful partnership:...
-
Product Partner laboratories
BioChem Labor für biologische und chemische Analytik GmbH offers a wide range of services which includes partner laboratories. Features: Identification of impurities, extractables / leachables, test for pyrogens in rabbits according to Ph Eur.. 2.6.8 / USP, test for abnormal toxicity Ph. Eur. 2.6.9 / USP, ...
-
Product Mérieux NutriSciences | Pharma & HealthCare complete services
Download our brochure: https://bit.ly/4dzryoA
Our complete range of Pharma services include: • Quality controls • Investigation studies • Absorption studies • R&D and validation activities • Stability & storage • Cleaning and disinfectants validation • Environmental monitoring
-
Product Commercial Mass Spectrometry Services
Mass spectrometry is among the most powerful analytical techniques available for protein characterization. KBI’s state-of-the-art mass spectrometry core facility delivers unparalleled structural characterization services to our clients.
Our expert team brings decades of experience in protein and p...
-
Product Analysis Service
Lomapharm GmbH provides wide range of services including analysis service. It involves chemical and physical analysis of raw materials, intermediate and finished products according to national and international specifications as well as customer-specific information (microbiological purity, stability t...
-
Product LNPs production services by microfluidics
We offer method development services for the production of lipid nanoparticles LNPs using the microfluidics platform Sunshine from Unchained Labs.

-
Product Drug Substance Services
CARBOGEN AMCIS provides comprehensive Drug Substance services, offering expertise in process development, scale-up, and manufacturing of active pharmaceutical ingredients (APIs). With state-of-the-art facilities, the company specializes in high-potency compounds, including cytotoxics, and delivers solu...
-
Product Hexane(HPLC grade)
Package size including 1L/bottle,2.5L/bottle,4L/bottle.Always keep 1000bottles in stock.
-
Product Analytical Development
BioDuro-Sundia’s Analytical Testing team offers high quality analytical services including method development and validation, qualification of reference standards, testing and release studies, stability studies, and CMC dossier preparation services. ...
-
Product Recipharm Analytical Solutions™
Through Recipharm Analytical Solutions™, we support customers with stand-alone analytical requirements. Our analytical development team has experience from developing hundreds of analytical methods every year, supporting development of formulations ranging from powder in capsules and IV solutions to ER tab...
-
Product Analytical Services
Our Analytical Services encompass critical aspects of drug development and manufacturing, beginning with Analytical Testing to ensure product quality, employing advanced instrumentation and experienced teams to facilitate compliance. We excel in Method Development & Validation, utilizing HPLC and UHPLC...
-
Product R&D service
Neotron Pharma is able to offer you a research and development service. The research and development laboratory, operating under the ISO regime, will be able to develop customized methods for the customer in faster times and with lower costs. Subsequently, the customer will be able to validate the GMP meth...
-
Product Services - Lab analysis
We offer the quality standards that we apply to our own environment, machines, solutions and processes as services to our customers as well.
We make continuous investments in our specialist expertise. Quality assurance is the focus of our work. To ensure that we can meet your requirements r...
-
Product Laser Micromachining Services & Systems
From pinholes in stainless steel discs to complex arrays in semiconductor guide plates, we provide a wide range of ultra-high precision laser drilling, cutting, milling, scribing and ablation contract services (and systems) for numerous applications in industry & academia. With over 45 years of experie...
-
Product Analytical Techniques
Comprehensive analysis during formulation development and GMP manufacture is vital to ensure that your drug has optimal delivery properties and stability profile as well as supporting your regulatory submissions.
-
Product Excipients Formulation Development Support
Excipients play a central role in the drug development process, in the formulation of stable dosage forms and in their administration. Though excipients were at one time assumed to be “inactive” ingredients, it is now understood that excipients can have an important impact on the pharmaceutical...
-
Product Pantoprazole Sodium
It is a digestive system medication suitable for the treatment of acute upper gastrointestinal bleeding such as duodenal ulcers, acute gastric mucosal lesions caused by gastric ulcers, and compound gastric ulcers. -
Product Quality Control
Our quality control department offers a broad range of services from incoming goods testing, in process controls to release of final product and stability testing. Our workflow ensures that all products are safe for market release and simultaneously functions as a feedback tool to help in the optimizat...
-
Product Research & Development
Discovery - Biology, Lead Optimization, Libraries, Synthetic & Medicinal ChemistryDevelopment - Chemical Process R&D, Fermentation, Formulation Development, High Potency, Kilo Lab & Small Scale Manufacturing, Lipid Nanoparticle, Method Development/Material Science, Rare/Orphan, Separation Scien...
-
Product Analytical Services - Inhaled Products
With extensive, state-of-the-art analytical testing facilities and equipment, our expert teams are able to develop and validate the methodologies required to characterise inhaled delivery platforms, especially DPI, pMDI and nebulised products.
To ensure seamless support for your development...
-
Product Isolation and Characterization of Product-related Impurities
Intertek offers product-related impurity analysis in line with ICH Q6B with laboratory-scale isolation and a range of chromatography or mass spectrometry approaches. Our experienced scientists perform detailed characterization using a diverse range of technologies which include MALDI-MS, LC-MSMS, HPLC...
-
Product European Pharmacopoeia Supplements 11.6 to 11.8
The 2025 subscriptions to the European Pharmacopoeia (Ph. Eur.), including the Supplements 11.6 to 11.8, are now available for purchase in the webstore. Two subscription formats are available: print and online versions.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance
_pages-to-jpg-0001.jpg)
_pages-to-jpg-0004.jpg)
_pages-to-jpg-0006.jpg)