Medical Devices

Product Description
Tentamus Group

-
DE
-
2017On CPHI since
-
1000 - 4999Employees
Company types
Primary activities
Categories
Specifications
Tentamus Group

-
DE
-
2017On CPHI since
-
1000 - 4999Employees
Company types
Primary activities
More Products from Tentamus Group (6)
-
Product Pharmaceutical Products
Bringing a pharmaceutical product to the market entails many complex, time consuming and expensive activities. Analytical expertise is a cornerstone in this development process. Tentamus Pharma Labs can support our customers throught the entirety of the development process and commercialization by prov... -
Product Biomolecules and ATMPs
Biomolecules like antibodies have revolutionized the pharmaceutical industry during the last decade and new products (e.g. Advanced Therapy Medicinal Products – ATMPs) are evolving in clinical studies and on the market. Tentamus Pharma Labs are among the leaders of analytical support for these product ... -
Product Microbiology
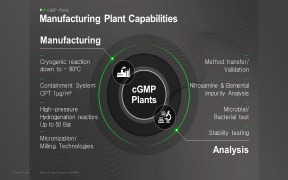
Microbiological tests are mandatory for non-sterile and sterile pharmaceutical products as well as medical devices. Tentamus Pharma Labs provide all standard test methods in this field and support your products in development or commercial phases. For non-sterile products, microbial enumeration tests (... -
Product Instrument-Based Analytics
The pharma laboratories within the Tentamus Group are equipped with state of the art analytical instruments offering an unparalleled array of analytical and bioanalytical services. The range covers simple Pharmacopeia methods (e.g. Ph.Eur, USP) to very complex analyses of biomolecules. We offer all kin... -
Product Physico-Chemical. Analysis
Physico-chemical methods are required in a range of products and compounds such as excipients, API’s, bulk material and finished products. Product characterization methods are often mandatory requirements within compendial monographs e.g Ph.Eur, USP, JP, BP. etc. as well as individual Marketi... -
Product Medical Cannabis
Medical Cannabis is a rising star in the pharmaceutical arena. Research indicates, that it might open new ways in alleviating and treating severe conditions in a variety of indications. One of the biggest challenges for companies in the field are regulatory and analytical requirements of the pharmaceut...
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance















.jpg)







-comp246698.jpg)