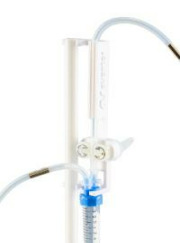
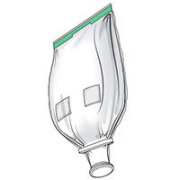
Avantor® Magnetic Mixing System
Product Description
Avantor

-
US
-
2022On CPHI since
-
4Certificates
-
5000+Employees
Company types
Primary activities
Categories
Specifications
Avantor

-
US
-
2022On CPHI since
-
4Certificates
-
5000+Employees
Company types
Primary activities
More Products from Avantor (5)
-
Product J.T.Baker® Cell Lysis Solution and J.T.Baker® Endonuclease
J.T.Baker® Cell Lysis Solution and J.T.Baker® Endonuclease - two innovative products that together sustainably optimize the gene therapy harvest process. Readily biodegradable and REACH compliant J.T.Baker® products offer a scalable and sustainable clarification process. Better together, these highly effec... -
Product Avantor OmniTop Sample Tubes® adjustable volume sampling system (AVSS)
AVSS helps our customers overcome complex scale-up challenges, particularly in sensitive applications like cell and gene therapy production and mAbs downstream processing. The single-use sampling device, with reusable adjustment tool and rack, can easily be used stand alone or configured in a manifold ... -
Product J.T.Baker® Direct Dispense packaging system
Enhance the effectiveness of your products and streamline your biopharmaceutical manufacturing processes with the J.T.Baker® Direct Dispense packaging system. This novel packaging platform makes it easy to deliver powdered performance materials and excipients directly to your process, whether you are using... -
Product J.T.Baker® Viral Inactivation Solution
J.T.Baker® Viral Inactivation Solution Biotech Reagent is a highly effective, ready-to-use product, specifically developed for detergent-based viral inactivation in biopharmaceutical operations and plasma derived therapy processes. J.T.Baker® Viral Inactivation Solution is formulated with non-ionic deterge... -
Product MasterSense™ Smart Peristaltic Pumps
NEW integrated sensor technology, intuitive touchscreen and MasterflexLive® remote monitoring for the biopharma industry of tomorrow
Avantor resources (17)
-
Video Viral Clearance Breakthrough: Enhancing Efficacy and Protein Stability With a Regulatory Compliant, Novel Detergent
Effective viral clearance processes during biologics manufacturing are the final shield to ensure product and patient safety. One of the classical methods for viral inactivation (VI) is the detergent treatment step in manufacturing of various drug modalities, including antibodies, plasma-derived products and AAV. In this presentation, we will discuss the application of an environmentally safe and sustainable alternative for Triton X-100 to enhance VI while maintaining compliance with regulations. An extensive study with different model viruses demonstrated its capability of providing >6.5 Log of viral inactivation and high virus killing kinetics independent of operational temperatures (4 ⁰C to RT) will be shown. Health-based exposure information will be presented for use in determining operator and patient safety. Other benefits of using this detergent include no impact on product stability over typical operating durations and sensitive molecules such as bi-specifics or on process performance. This detergent can easily be removed from the process by typical chromatography techniques and be detected by a simple analytical assay. -
Video Panel – Bringing Academia's Technological Insights to Biomanufacturing
Join our panel of experts as we delve into the latest innovations and advancements in academia, exploring how these developments can benefit the biologics industry. -
Video Pharma Awards Winner - KinetiSol(™) Technology
Join our Pharma Awards Winner as we Recognise their achievements in accelerating innovation and improving patient outcomes across healthcare -
Video Getting Your Research Published in Medicine & Health Journals: The Do's and Don'ts
What does the publication process look like? How to prepare a manuscript Ethical considerations during the publication process Identifying the target journal Peer review do’s and don’ts How we can help you enhance your research through our services -
Video Optimising Biosimilar Use for a Sustainable Healthcare Future
Biosimilars are delivering on their cost-saving potential, reducing biologic medication costs and expanding treatment options. However, there are hurdles that hinder optimal biosimilar use, potentially jeopardizing their long-term viability in the healthcare system. Examining these challenges through real-world examples and case studies is essential to ensure biosimilars continue to play a significant role in overall healthcare affordability and access. -
Video Exploring Emerging Innovations in Oncology
Oncology has always been a powerhouse of innovation in healthcare, and with ground-breaking new technologies on the horizon, its impact is only set to continue. This dynamic session will go deep into growth areas within Oncology and evaluate the future pipeline and its implications in: Elevating patient outcomes to new heights Successfully launching medicines Starting up clinical trials Expanding manufacturing capabilities Navigating off-patent opportunities Join us for an overview of Oncology's competitive landscape to gain a deeper understanding of the growth drivers in this fast-paced therapy area. -
Video Case Study: Can Cultivated Meat Revolutionise Biologic Manufacturing
Cell-cultured meat production is increasing, predicting to reach $1.1 billion in value by 2034. Though not the usual modality, various companies in the life science industry have expanded their interest and activity to cell-cultured meat research and manufacturing because of the similarity in equipment and expertise required. Exploring existing biomanufacturing facilities setup and the possibility to adapt cultivated meat production Comparing the regulatory frameworks for biologics and cultivated meat, highlighting key differences and potential challenges. Assessing the possibility of using cultivated meat technology to manufacture specific proteins or biomaterials for therapeutic purposes. Looking at the potential opportunities and challenges of scaling cultivated meat production to meet market demands compared to smaller-scale biopharmaceutical production. -
Video Navigating Tech Transfer in Biologics Manufacturing
This session dives into the critical stages of technology transfer, exploring key factors that empower manufacturers to streamline biologics production and ensure timely delivery of these life-saving therapies. How timely tech transfer can increase regulatory adherence Success factors for optimal tech transfer -
Video Optimising Biologics Production with Single-Use Technologies
Discover how single-use technologies are driving advancements in biopharmaceutical manufacturing. Exploring the advantages: Delve into the benefits of utilizing single-use technologies in biologics manufacturing, including increased flexibility, reduced contamination risks, and enhanced scalability. Case studies and insights: Gain valuable insights from real-world case studies showcasing successful integration of single-use systems with biologic production processes. Future outlook: Gain a glimpse into the future as we discuss emerging trends and developments shaping the ongoing evolution of the biologics-single-use partnership. -
Video Buffer Management Strategies: How to Stay Nimble and Competitive in Biologics Manufacturing
Biopharmaceutical development and production requires the investment of significant time and resources. Manufacturing procedures must be efficient, robust and productive to minimize failure risk and ensure targets are met. With increasing product titers, greater volumes of process buffer are required to meet processing demand. Increased buffer volumes place additional pressure on buffer hold and storage area constraints, particularly for companies that prefer to prepare their buffers in advance. Complex scheduling of staff and equipment is necessary to ensure there is an adequate quantity of QC-released process buffers prepared and ready for an entire batch. These logistical challenges increase in plants producing multiple products due to the need for different buffer solutions for different process applications. Furthermore, continuous manufacturing requires different considerations, as it requires constant supply of process buffers. Identifying the right buffer preparation strategy for a given facility and operation is key. While holding larger buffer quantities might be suitable for a fed-batch processes, in-line dilution or conditioning deserves careful evaluation for continuous manufacturing. Operational modifications in buffer management allow manufacturers to maximize their output without significantly altering their production approach. This can enhance several measures of efficiency, including faster drug product release, increased productivity of GMP manufacturing, reduced footprint, enhanced flexibility and improved quality. In this presentation, we will take a holistic approach to buffer management, discuss the known and unknown challenges, and consider different options of buffer preparation, weighing the merits of in-house vs. outsourcing. Conceptual design scenarios in both existing and new facilities will be shared to assist with the economics and logistics of the buffer journey. And finally, we will show how the best buffer management approaches come together to deliver significant operational benefits —allowing biologics manufacturers, including CDMOs, to remain nimble and competitive. -
Video Scaling Cell Therapy Manufacturing
Looking at examples of challenges and bottlenecks in cell therapy applications and what can be done to overcome them Assess automation and process optimization tools and its role in scaling up cell therapy production -
Video Panel – Driving Next-Gen Biomanufacturing with Digital Transformation
Digital transformation is becoming an essential part of biopharma manufacturing as it helps improve product quality and robustness. Join our panel of experts where we Exploring the power of digital technologies for real-time monitoring and quality enhancement in manufacturing Unveiling AI's expanding role in manufacturing and predictive maintenance Addressing challenges in upskilling and regulation to help maintain high standards across manufacturing -
Video Fireside Chat: Exploring the Microbiome’s Multifaceted Role in Future Therapies
Understand the potential of the microbiome in shaping the future of therapeutics. Dive into the latest technological innovations propelling microbiome research forward, uncovering new insights and possibilities. Discover the latest research findings with the microbiome Exploring microbiome influence in alternative therapeutic areas such as Neurology and Oncology -
Video Panel: Global Trends in Bio-Manufacturing
Bio-manufacturing is rapidly evolving with increased outsourcing, new modalities, and M&A opportunities shaping the landscape. Join our panel of experts and explore the latest in outsourcing trends, partnership models, and the M&A activity that is fostering growth, capacity and capabilities.. The outlook for outsourcing New modalities Partnership models M&A opportunities -
Video Keynote: Advancing Immunisation: Next-Gen mRNA Vaccines
Delve into the future of vaccination technology as market experts Moderna unveil the potential of mRNA vaccines to revolutionise healthcare. Don't miss this opportunity to explore the forefront of medical innovation and discover how these next-generation vaccines are reshaping the landscape of disease prevention. -
Video Keynote: The EU’s Biotech & Bio-Manufacturing Strategy
The European Commission published a communication launching the EU Biotech and Biomanufacturing Initiative to enhance competitiveness and industrial modernization. These efforts seek to foster innovation, facilitate investment, and position the EU as a leading hub for biotechnology and biomanufacturing. The initiative proposes eight key actions, including most notably: Launching a study to support the simplification and streamlining of the EU regulatory framework for biotech (potentially leading to an EU Biotech Act) Establishing an EU Biotech Hub to support navigation of the regulatory framework and scale up Setting up several mechanisms to increase funding and investment in EU biotech and biomanufacturing Reviewing the EU Bioeconomy Strategy These actions have significant implications for stakeholders within the EU life sciences ecosystem—especially in the context of the ongoing Pharma Package -
Video Fireside Chat – Improving Patient Access with Biosimilars
Join us on day 2, into the potential of biosimilars to expand access to critical treatments for patients. We'll explore how these medications can play a significant role in healthcare, while also discussing the opportunities and challenges associated with their wider adoption.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance








-file153345.png)
-file153430.png)
-file153429.png)
-file153428.png)

-file153363.png)
-file153362.png)

-file153360.png)
-file151698.png)

-file153342.png)


-file153338.png)
-file153337.png)
