Residual solvents

Product Description
Neotron Pharma SpA

-
IT
-
2016On CPHI since
-
3Certificates
-
500 - 999Employees
Company types
Categories
Specifications
Neotron Pharma SpA

-
IT
-
2016On CPHI since
-
3Certificates
-
500 - 999Employees
Company types
More Products from Neotron Pharma SpA (10)
-
Product Extractables & Leachables
There are many contaminants that could be released inside a drug during the production process or by contact with the packaging material. Neotron Pharma will be able to support you from the study of Extractables, to the toxicological evaluation up to the control of the Leachables. What distinguishes us is ... -
Product Pharmacopea Analysis
Neotron SpA provides wide range of analytical control according to EP, USP, JP, BP, CHP.
Contact us to ask for tests in relation to your monograph of interest. -
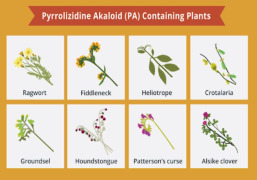
Product Pyrrolizidine Alkaloids
Neotron Pharma has an HPLC-MSMS method for the determination of 28 pyrrolizidine alkaloids on different matrices. We currently collaborate with numerous herbals producers for these routine checks for both the Pharma and Food markets -
Product Contaminants
Neotron Pharma, thanks to its many years of experience in the Food department, is able to quantify the presence of contaminants such as Pesticides, Aflatoxins, Mycotoxins under GMP regime. This type of analysis can be fundamental for all those suppliers of raw materials and Erbal intended for the supplemen... -
Product Method Validation
Neotron Pharma is able to validate analytical methods developed internally or transmitted by the customer using top of the range equipment such as:
- HPLC-UV / DAD / MSMS
- GC-FID / ECD / MSMS (even in Headspace)
- ICP-MS / AAS / AES / OES
- Post-column derivatization
- Particle si... -
Product R&D service
Neotron Pharma is able to offer you a research and development service. The research and development laboratory, operating under the ISO regime, will be able to develop customized methods for the customer in faster times and with lower costs. Subsequently, the customer will be able to validate the GMP meth... -
Product Stability tests according to ICH
Neotron Pharma provides Stability tests according to ICH standards (zones I, II, IV). We can carry out stability studies in their entirety (storage + analysis) or provide only storage service. The conditions we can offer are listed below:
- 40°C ± 2°C , 75% ± 5% U.R.
- 30°C ± 2°C&n... -
Product Narcotic Substances
Neotron Pharma is able to perform all the analytical techniques requested by the customer on APIs or finished products subject to restrictions as they are part of the narcotic substances. The laboratory will be able to verify if your specific active ingredient is free to manipulate or subject to restrictio... -
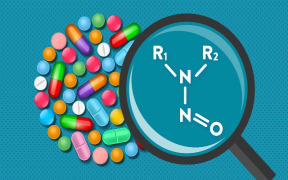
Product Nitrosamine impurities
Neotron Pharma, on request of several customers, has provided an effective methodological approach for Nitrosamine alerts management to support the pharmaceutical industry. Currently the laboratory is able to perform screening and validation activities for more than 11 Nitrosamine residues in API, FP and e... -
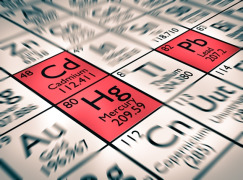
Product Elemental Impurities
In light of the growing interest of the pharmaceutical world on the issue of determining Elemental Impurities in accordance with tables 1, 2a, 2b and 3 of the ICHQ3D, Neotron Pharma laboratory, thanks to its decades of experience in the analysis of metals, has developed a series of analytical proposals to ...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance