INCOG BIOPHARMA SERVICES
About INCOG BIOPHARMA SERVICES
Certifications
Categories

-
US
-
2022On CPHI since
-
1Certificates
-
50 - 99Employees
Company types
Primary activities
Meet us at
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025
CPHI Frankfurt 2025
Messe, Frankfurt
28 Oct 2025 - 30 Oct 2025
Products from INCOG BIOPHARMA SERVICES (2)
-
Product Analytical Services
Analytical Services: QC Chem/Microbio, stability, etc.
-
Product Drug Product Fill-Finish Services
Fill-Finish Capabilities (product type) and Capacity:
Sterile liquid products
Biosafety level (BSL) 1, BSL 2 (OEL >= 10 µg/m³)
Vaccines (inactivated)
Current capacity: ~40M units/year
INCOG BIOPHARMA SERVICES Resources (2)
-
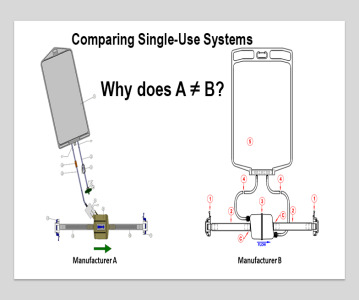
Sponsored Content Building resiliency into single-use systems
Jo Anne Jacobs, Director of Technical Services at INCOG BioPharma Services, discusses building a functional equivalence model for single-use manufacturing equipment and assemblies for use in drug product manufacturing. -
News INCOG BioPharma Services completes analytical and quality control testing laboratory and technology transfer suite
In February, INCOG BioPharma Services completed its 6,000 sq. foot laboratory space at its Fishers, Indiana headquarters
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance