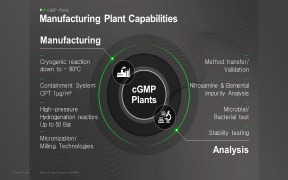
Quality control laboratory
Product Description
Laboratori DAU

-
ES
-
2022On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
Primary activities
Categories
Specifications
Laboratori DAU

-
ES
-
2022On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
Primary activities
More Products from Laboratori DAU (2)
-
Product Packaging
PACKAGING PHARMA - GMP SERVICES • Primary packaging of solid oral dosage forms • Secondary packaging of all pharmaceutical forms • Serialization of commercial kits and clinical packaging • Re-Packaging and batch quality review • Cold chain in secondary packaging and re-packaging... -
Product Packaging and Labelling for Clinical Trial Products
SERVICES • Primary packaging of solid oral dosage forms • Secondary packaging and labelling of all pharmaceutical forms • EU GMP batch qualification • Batch certification for clinical trials • Import and export services • IMP distribution and collection - GDP compliance • Reconciliation and Destructi...
Laboratori DAU resources (1)
-
Brochure PRODUCT SERVICES
With more than 25 years of experience, we have a 6,800 m2 facility located in Zona Franca - Barcelona.
QUALITY CONTROL LABORATORY SERVICES:- Analysis certificates: physical-chemical, microbiological, sterility and endotoxines
- Release certificates: QP declaration
- Import / Export of pharmaceutical products
CLINICAL TRIAL PRODUCTS
- Primary and secondary packaging- Labelling processes
- Supply of packaging materials
- Randomization - Blinding
- EU GMP Certification
- Quality Control Analysis
- Batch import and export
- Distribution
- Reconciliation and destruction
- Cold Chain (2 - 8ºC)
PHARMA PACKAGING
- Primary packaging of solid forms
- Secondary packaging of all pharmaceutical forms
- Serialization of standard and big sized boxes
- Authorisation for psychotropic and narcotic medicinal products
Recommended Products
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

















-comp246698.jpg)