All News
All news
-
News Pharmapack 2021 Packaging and Drug Delivery Trend Report: Out Now!
Rather than looking back at what has been a challenging past few months, this Trend Report - featuring highlights from the Pharmapack 2021 content programme - has been written very much in the spirit of looking forward and addressing the key challenges... -
News Increasing demand for outsourced manufacturing due to changing Chinese market conditions, CPHI Worldwide conference told
Rise of innovative companies with high-risk portfolios will necessitate need for CDMOs, says Thermo Fisher senior executive -
News Catalent embarks on $230M viral vector expansion project at Harmans campus
By completion at the end of 2022, the campus will accommodate 18 cGMP viral vector manufacturing suites -
News API supply chain needs to address future challenges arising from China situation: CPHI Worldwide panel
COVID-19 pandemic has exposed weaknesses and sector faces new challenges driven by rising costs of production in China -
News Digitalisation the key to efficient EU pharmaceutical regulation and easing of localised drug shortages
Current EU regulatory systems too overwhelmed by paper, head of European association representing generics, biosimilars and value added medicines industries tells CPHI Worldwide online conference -
News Indian pharma industry on cusp of growth spurt, CPHI Worldwide audience told
Country expected to grow threefold over the next decade due to increased focus on complex generics, biosimilars and specialty products -
News GTP Bioways to galvanise CDMO position in France with two new biopharma facilities
One site will focus on microbial production, the other on small-scale mammalian-based production -
News Next stop, Milan - Welcome to Italy's life sciences hub
Milan is home to an internationally competitive pharmaceutical, biotechnology and functional foods hub, with strong growth prospects and a commitment to innovation across its entire supply chain. Italy is also home to one of the most advanced and ... -
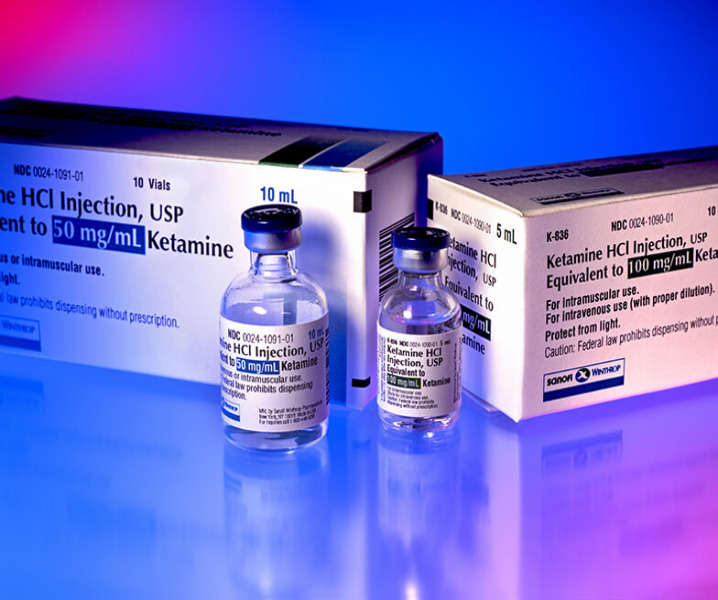
News Alcami to manufacture PharmaTher’s proprietary ketamine products
The CDMO will supply expertise in GMP sterile fill-finish manufacturing and controlled-substances -
News Phlow receives approval to join BARDA CDMO network
As a network member, the company hopes to help reduce the US's reliance on overseas imports by providing domestic API capability -
News Novavax defends COVID-19 vaccine manufacturing processes after claims of quality issues
According to unnamed sources in Politico report, company’s vaccine batches have not met FDA purity level standards -
News FDA misses approval target dates after plant inspections delayed by COVID travel restrictions
UCB Pharma is informed by agency that action on application for psoriasis treatment is being deferred until on-site visits can be made -
News FDA declines approval of United Therapeutics’ Tyvaso after inspection issue
In complete response letter, agency cites single deficiency at third-party facility that performs analytical testing of Treprostinil drug substance -
News Matica Bio and Sartorius form large-scale vector production research pact
Together the companies will attempt to overcome "black box" challenges to enable more robust and consistent results for clients -
News China SXT's new lyophilisation facility secures pharmaceutical manufacturing permit
The move, coupled with the plants's passing of a pharmaceutical GMP compliance inspection, will enable company to accelerate the R&D of its novel Advanced TCMPs -
News Avid Bioservices to plug capacity gap with new viral vector manufacturing facility
The biologics CDMO says it expects its new 53,000-sq.ft facility will be operational within 18 months -
News Everest Organics starts API production for Merck anti-COVID pill
Manufacturer starts development of API for molnupiravir, which is set to become world’s first oral treatment against coronavirus -
News Pharmapack Europe 2021 announces Awards winners!
Awards showcased a wide range of industry-changing innovations in packaging and drug delivery, with sustainability emerging as a key metric across all categories -
News Pharmapack 2021: Barriers to healthcare adoption of growing sustainable packaging trend still exist, say experts
Panel discusses how healthcare institutions are adapting to calls for more sustainability in various processes in the value chain -
News Aceto boosts biomanufacturing footprint with acquisition of Ireland-based A&C Bio Buffer
The transaction expands the CDMO's GMP biopharmaceutical and vaccine manufacturing capabilities -
News Merck KGaA doubles gene therapy capacity with opening of second Carlsbad CDMO facility
The new the 140,000-sq. ft facility will support commercialisation and industrialisation of viral vectors -
News Eckert & Ziegler expands CDMO services to support radiopharma companies
Having acquired a 53,000 sq-ft property in Berlin, the company plans to set up new laboratories, production and cleanrooms -
News CPHI Webinar: mRNA vaccines, Trends, Technologies and Supply Chain
The COVID-19 pandemic has meant scientists, together with pharmaceutical companies, have had to reinvent how to bring vaccines to market faster without impacting product quality, safety, or efficacy, according to experts speaking at a recent CPHI webin... -
News Pharmapack 2021: Pre-filled syringe technology could provide answers to future mass vaccination challenges, says BD
Pre-filled syringes (PFS) could help immensely in solving the challenges of rolling out future mass vaccination campaigns, according to experts at Becton Dickinson (BD). -
News Pharmapack 2021: Increasing connectivity is the key to patient centricity and simplified access to product information
During the live Innovation in Connectivity Roundtable at the Pharmapack 2021 online conference, experts discussed how the latest innovations in digital technology are shaping industry ambitions to put the patient journey at the heart of its drug device... -
News Boehringer opens doors to new Austrian biopharma production site
The LSCC provides an additional 30% in mammalian large-scale capacity for the company's own pipeline and for third parties -
News COVID-19 triggers growing interest of European patients in where drugs are manufactured: Teva study
Just as 'food miles' have become a key concern among consumers in recent years, patients now want to know more about 'medicine miles', study commissioned by Teva finds -
News Pharmapack 2021: Convergence of trends are pushing drug delivery devices towards connectivity, says Nemera
Electronic devices and connectivity are being progressively included in drug delivery devices, bringing positive outcomes to all stakeholders, according to drug device combinations specialist, Nemera Tuesday -
News Pharmapack 2021: Traceability of medicines can transform pharma supply chain, delegates told
Traceability of medicines can help to reduce the negative effects to pharmaceutical supply chains of counterfeit medicines, medication errors and drug shortages, Nathalie Wardé-Dunoyer, CEO & Founder of Swiss start-up, D4P pharma told the Pharma... -
News Iovance opens PA cell therapy plant in Philadelphia
The $85 million Iovance Cell Therapy Center (ICTC) has opened its doors at the Philadelphia Navy Yard with capacity to supply thousands of patients per year. -
News Advanced Computing in Pharma: 3 reasons why quantum computing could disrupt R&D
Quantum computing technology uses quantum systems called “qubits”. They work with non-binary values, unlike classical computing which uses only bits with values of 0 or 1, and can overlap each other and act as a group. The result is a ... -
Sponsored Content Thermo Fisher Scientific Opens cGMP Plasmid DNA Manufacturing Facility in Carlsbad, California
Responding to critical market capacity constraints, Thermo Fisher announces the official opening of new 67,000 sqft cGMP plasmid DNA manufacturing facility in Carlsbad, CA. This site expands clinical and commercial capabilities for cGMP plasmid DNA use... -
News Forge Biologics to provide CDMO support for Solid Biosciences' preclinical gene therapy
By providing AAV process development, scale up and cGMP manufacturing services, Forge hopes to bolster Solid's gene therapy pipeline -
News MannKind signs sale-leaseback agreement for inhaled insulin manufacturing site
The transaction will generate $102.25 million to support the company's product pipeline and scaling up commercial activities for Afrezza -
News Greenfield Global Ireland Ready to Supply Life Science Customers Globally
New facility aims to serve local and global pharmaceutical customers with high-purity ethanol, ethanol blends and buffer solutions -
Sponsored Content IFF Pharma Solutions: Paving the Way for Superior Suspensions
Shawn Branning, Global Strategic Marketing Manager at IFF – Pharma Solutions -
Sponsored Content CPHI Webinar: Develop ODTs in a FLASH with UltraBurst™ - Watch On Demand
Catch up on the latest trends and developments in orally disintegrating tablet (ODT) formulation development -
Sponsored Content Biocatalysis: An Indispensable Tool for Synthesis of Active Pharmaceutical Ingredients
Biocatalytic processing offers a wealth of advantages and should be evaluated during early-stage drug development for its potential to improve yields, increase efficiency, and support greener manufacturing. -
News Fujifilm begins commissioning phase of new Netherlands-based manufacturing facility
The facility will increase the production capacity of cell culture media products and provide a hub for European and global markets -
News Rare diseases care pathway: a major challenge to harmonize patient management
In France, rare diseases are numerous: between 7,000 and 8,000 diseases are currently accounted for, and concern more than 3 million French people, accounting for almost 4,5% of the total population. The organization of screening and care for these ill... -
News Sartorius to add flagship site in North America
The company plans to open a new 130,000-sq. ft plant in Ann Arbor in late 2023 -
News Hikma boosts US injectables business with $425 million deal for Custopharm
The acquisition will add 13 approved products and additional pipeline products -
News Report accuses Covid vaccine makers of putting profits before lives
Only 0.3% of the 5.76 billion doses administered worldwide have been distributed to low-income countries -
News SK Capital Partners in discussions to create an integrated global API CDMO leader
The private investment firm plans to acquire SEQENS and merge it with Wavelength Pharmaceuticals -
News 5 Highlights from the Pharmapack Europe Content Programme
Pharmapack Europe is back! Join us online from 27 September as we kick off our two-week long content programme, a great precursor to the in-person event from 13-14 October at the Paris Expo Porte de Versailles. -
News Fresenius Kabi invests in Pre-Filled Syringe Capacities for US and EU supply
As a safe, effective and convenient way to administer injectable medications, pre-filled syringes (PFS) have become the injection system of choice for a growing number of drugs. PFS allow healthcare professionals to easily handle syringes, reduce waste... -
News New Thermo system makes AAV production more efficient and scalable
The company's new Gibco AAV-MAX Helper Free AAV Production System helps reduce production costs and streamline transition from research to clinical environments -
News Catalent and Phathom Pharmaceuticals strike commercial supply agreement
The CDMO will provide commercial manufacturing and packaging services for Phathom's novel, orally active P-CAB -
News Catalent boosts nutraceuticals market presence with $1 billion Bettera acquisition
The transaction is expected to accelerate Catalent's growth in global softgel and oral dose formulation and manufacturing services -
News Introducing the all-new CPHI Online dashboard
Today, Informa Markets is pleased to announce the launch of the all-new CPHI Online Dashboard, which will allow CPHI clients to quickly and easily track awareness and interest in their company, products and content as well as view and download leads to... -
News Clean Cells to invest €13 million to create Europe's largest cell bank production site
The expansion is anticipated to speed up development and time-to-market of novel treatments and vaccines for COVID‑19 -
News AGC Biologics to increase capacity for pDNA and mRNA manufacturing in Heidelberg
The expansion plans include an additional manufacturing line, a new cleanroom and a new process development lab -
News Room for specialists and one-stop-shops in CDMO space
As CDMOs seek to diversify their business models, is a full-service offering the holy grail or is there a danger of biopharmaceutical outsourcing firms becoming the jack of all trades and the master of none? -
News Thermo Fisher to build facility in Nashville for production of single-use technologies products
Investment will further expand CDMO's global network of SUT facilities to boost reliable supply of critical materials used to produce new biologics and vaccines -
Sponsored Content CPHI Podcast Series: Selecting the Right CDMO Outsourcing Partner
Sponsors within the biopharmaceutical industry are increasingly turning to outsourcing for several reasons; remaining competitive and flexible within a quickly evolving sector, gaining access to manufacturing capacity and state-of-the-art equipment and... -
News Emerging regional markets show promise as CDMO globalisation trend grows
Opportunities for CDMO expansion are extending to Latin America and Africa, say experts -
News GenScript ProBio to manufacture clinical trial batches of Synimmune's lead antibody drug
The CDMO will manufacture drug substance and drug product for Phase II clinical trials in AML patients -
News Takeda to supply Japan with 150 million doses of Novavax COVID vaccine
Japan's biggest drugmaker prepares to make the vaccine domestically and anticipates early 2022 distribution -
Sponsored Content Clinical & Commercial Drug Product Manufacturing: Outsourcing Advice from a CDMO
Taking a sterile drug product from clinical to commercial scale brings unique challenges. In this article, LLS Health investigates ways to mitigate scale-up challenges and achieve success. -
News Formulated Solutions completes investment and expansion project
The CDMO significantly increases its footprint, capacity and capabilities to meet current and future demand -
News 3i Group backs ten23 health to create global CDMO focused on biopharma injectables
The new Switzerland-based CDMO differentiates itself by its sustainable vision of making a positive impact for people and planet -
News Thermo Fisher Scientific and AstraZeneca sign NGS-based CDx co-development agreement
Companies say earlier collaboration may speed development and introduction of targeted precision medicine therapies for patients. -
News Reopened USAntibiotics penicillin facility to relieve US dependence on Chinese antibiotics: Jackson Healthcare
The facility will be able to manufacture and stockpile a five-year supply of amoxicillin for the US -
Sponsored Content Sterile manufacturing Annex 1 amendments: what, why and how explained
In January 2020 the European Commission published some proposed significant revisions to Annex 1, the EU’s good manufacturing practice (GMP) guide for the manufacture of sterile products. In the following three-month consultation period, affected... -
News Cipla and Kemwell to form joint venture targeting respiratory biosimilars market
The agreement is expected to accelerate Cipla’s global lung leadership agenda -
News Medicines Manufacturing Innovation Centre aims to 'solve major pharma industry challenges'
£35 million public-private collaboration to develop next-generation pharmaceutical manufacturing processes to drive forward innovation in the medicines supply chain -
News Recipharm expands its analytical services offering in India
The CDMO inaugurates its new analytical laboratory under Recipharm Analytical Solutions -
News Signature Biologics opens additional manufacturing and R&D facility
The new facility will augment the company's capacity to broaden its portfolio of perinatal-derived products -
News Pfizer-BioNTech shot becomes first COVID-19 vaccine to gain full FDA approval
Pfizer and BioNTech’s shot on Monday became the first COVID-19 vaccine to be granted full approval by the US Food and Drug Administration. -
News Lonza to establish multi-product fill and finish line in China
The new production line at Guangzhou site will supply global and domestic companies with clinical and commercial batches -
News Responsible Supply Chain Practices: Highlights from the PSCI Annual Report
An update on responsible supply chain management from the Pharmaceutical Supply Chain Initiative Chair, Manjit Singh -
News CPHI Podcast Series: The opportunities and challenges of providing fill and finish services for mRNA platforms
mRNA (Messenger ribonucleuc acid) technology has been thrust into the limelight in recent months, with the platform proving to be the springboard for two approved vaccines against the SARS-CoV-2 virus. -
News Curia lays out plans to expand complex API manufacturing capacity
The $35 million investment will more than double the site’s batch-size scaling and product output. -
News Biotage expands production capacity to ease lipid bottlenecks
New site in Cardiff boosts the company's production capacity for equipment crucial in the supply chain for mRNA vaccines production -
News Tikun Olam-Cannbit and Wavelength strike novel cannabis agreement
Firms say the partnership will "multiply the power of both companies, the pharmaceutical industry and the medical cannabis industry" -
News PBS Biotech secures $10 million to expand cell therapy manufacturing equipment portfolio
The capital will be used to expand and industrialise the company's range of single-use bioreactor systems and contract process development services -
News Eliminating the Analytical Bottleneck in Production and Purification of mRNA
COVID-19 has focused a spotlight on the ability of mRNA technology to accelerate vaccine development and approval. That same technology can hasten the development and approval of other therapeutic classes, including cancer immunotherapy, protein replac... -
News Recro eyes expansion to full-service CDMO status
The solid oral dose specialist signs letter of intent for acquisition of a CDMO providing a full range of services -
News Moderna to build mRNA vaccine manufacturing facility in Canada
The new facility will provide access to domestically manufactured vaccines against respiratory viruses -
News COVID-19, patient needs and sustainability driving drug delivery tech innovation
Innovation is the cornerstone of the drug delivery technology sector; efforts to make therapies more convenient for patients, reduce side effects and differentiate products from the competition have always made the delivery sector a hotbed of new ideas... -
News 5 Things We Learned from the CPHI North America Content Program
The CPHI North America exhibition is taking place in-person this week in Philadelphia from 10-12 August. Here we take a look at key learnings from the past two weeks of expert-led content, available to all attendees as part of the hybrid event experien... -
News Bora Pharmaceuticals and KYOWA agree generic manufacturing contract
KYOWA Pharmaceutical Industry will be the CDMO's first Japanese client for its Zhunan manufacturing site -
News Diversifying Supply Chains — Does USMCA Offer New Opportunities for Pharma?
Dependence on Asia – and specifically China – has been the subject of a great deal of attention by political and industry leaders in the last year. In North America, this has led to calls to “reshore” drug production to domestic facilities. -
News Cosmetics packaging needs to respond to increasing consumer awareness of sustainability: CPHI Webinar panel
The cosmetics packaging sector will need to respond to increasing consumer awareness of climate change by transitioning from fossil-based materials to long-term carbon neutral and carbon negative alternatives, according to industry experts speaking at ... -
News Tech transfer: a many-layered artform that demands precision and communication
Tech transfer is a crucial phase that converts clinical promise into commercial gain but pressure on sponsors to reduce time to market in the age of COVID-19 means CDMOs have to be on top of their game to deliver. What are the latest innovations to sol... -
News DisperSol signs up Catalent for manufacturing of multiple pharma products
The collaboration will see Catalent install a commercial-scale KinetiSol technology manufacturing line at its Somerset, New Jersey, facility -
News WuXi STA completes acquisition of its first European facility
The purchase of BMS's Couvet, Switzerland facility adds commercial-scale production capacity for capsule and tablet dosage forms -
News Discover the CPHI North America Learning Labs: Part Two
Explore the series of Learning Labs at CPHI North America in which thought leaders at our exhibitors showcase their extensive knowledge on all areas of the pharma supply chain, offering industry insights across drug manufacturing, outsourcing, pharma i... -
News Viatris and Biocon score historic first for interchangeable biosimilar product
The new approval of Semeglee allows pharmacy-level substitution for Lantus across the US -
News Discover the CPHI North America Learning Labs: Part One
Explore the series of Learning Labs at CPHI North America across several product innovation categories in which thought leaders at our exhibitors showcase their extensive expertise in all areas of the pharma supply chain, offering industry insight... -
News CPHI Trend Report - CDMO Opportunities in the Chinese Market
Opportunities for contract development and manufacturing organisations (CDMOs) in China continue to grow but many questions remain over what the future of the sector will look like in the world's second largest pharmaceutical market. -
News Diversification and addition of capabilities key to CDMO success as end markets grow: CPHI North America panel
CDMOs need to diversify and expand their capabilities to take full advantage of strong growth in prominent end markets such as small molecule, monoclonal antibodies and cell and gene therapy, according to an industry expert on a panel at the CPHI North... -
News Sartorius extends its cell culture media business via acquisition
Acquiring Xell AG will enable Sartorius to offer specialised media for manufacturing viral vectors -
News US needs to look differently at public health preparedness, says former FDA commissioner Gottlieb
Speaking at CPHI North America, Dr Scott Gottlieb shares insights into how the US should prepare for future pandemics by building out vaccine capacities -
News Pandemic and transformative technologies fuel growth in pharma outsourcing sector: CPHI North America panel
Transformative technologies and the opportunities afforded by the COVID-19 pandemic have spurred investment in the life sciences outsourcing sector, according to a panel of industry experts speaking at CPHI North America on Monday. -
News Thermo Fisher pledges to reach carbon neutrality by 2050
Accelerated efforts align with the Paris Agreement and Race To Zero to combat climate change -
News Digital health tools ‘becoming integral parts of mainstream medicine’: IQVIA report
More than 350,000 health apps are currently available, increasingly focused on helping them manage their conditions rather than on wellness management -
News The latest trends for cosmeceuticals: what is fueling consumer drive towards beauty?
The rise in the concept of ‘self-care’ has forced the beauty and cosmetics industry to think differently about how they launch their products. Martin Oxley, Managing Director at buzzback explains why the cosmeceuticals sector does not just ... -
News CPHI Podcast: The distinction of drugs vs cosmetics from a regulatory perspective
As more and more companies operating in the pharmaceutical space are moving into cosmetics, the temptation is to think that life will be much easier from a regulatory and compliance perspective. -
News Catalent plans $100 million biologics manufacturing expansion at Anagni
Two 2,000-liter single-use bioreactors to be installed as part of first phase expected to be operational in April 2023 -
News Merck KGaA invests €270 million to create "site of the future"
The investment will fund the construction of a Translational Science Center for its Healthcare business sector, as well as a new Learning Center -
News BioNTech to purchase Kite manufacturing facility and cell therapy R&D platform
The acquisition will add manufacturing footprint in the US enabling the company to develop its pipeline of novel cancer product candidates -
News Samsung Biologics and Kineta strike cancer immunotherapy development and manufacturing deal
Samsung to provide end-to-end CDMO services to advance Kineta's novel anti-VISTA antibody for the treatment of solid tumours -
News CPHI Podcast Series: The Cannabinoid Opportunity for Cosmetics & Pharma
Cannabinoids have caught the attention of Pharma and Cosmetic companies alike in recent years, celebrated for their anti-inflammatory, anti-oxidant and anti-bacterial properties in cosmetic products, and neurological and chronic pain benefits on the ph... -
News Skyepharma selects Veratrak platform to ensure secure data storing and sharing
Using Veratrak's platform, the CDMO will have full oversight of data, including an audit trail of data and document processes -
News Thermo Fisher Scientific opens plasmid DNA manufacturing facility at Carlsbad campus
67,000-square-foot facility at Carlsbad campus is part of CDMO's investment strategy that addresses growing global demand for cell and gene therapies and vaccines -
News Curia to buy fellow CDMO Integrity Bio
Acquisition will add West Coast coverage to the CDMO's East Coast and European capabilities and enhance its biologics formulation development and fill-finish network -
News Catalent enters CBD anaesthetic product formulation project
The company will use its orally disintegrating tablet technology Zydis for JOS Pharmaceuticals' licensed CBD product -
News Sparx Therapeutics to build first commercial antibody drug production facility in China
The emerging biotech company unveils plans for a 1.2 million-sq. ft manufacturing facility in Yangzhou -
News LGM Pharma launches analytical and stability testing as standalone service
The integrated CDMO rolls out services to the broader community of drug manufacturers and developers -
News Pharmapack Podcast: Patient Partnerships for Medical Devices
Healthcare provision is constantly evolving. Whereas in the past, decision making on treatments for patients was very much the domain of physicians and doctors, patients now feel more empowered to be involved in the decision making process. The healthc... -
News Thermo Fisher supports gene developers with new AAV manufacturing solutions
A new media panel, gene kit and advanced resins help reduce manufacturing costs and increase the viability of gene therapies -
News Novartis shifts Leqvio manufacturing to Austrian facility
Pharma firm tells FDA tech transfer to its own facility in Schaftenau, Austria is underway after regulator highlighted concerns about third party CDMO site in Italy -
News Tipping the scales - Aduhelm’s hefty price under the spotlight
Biogen’s Alzheimer’s drug Aduhelm has recently secured FDA approval to much fanfare. But do the benefits of the treatment justify the weighty $56,000 per year price tag? -
News 5 Strategies for Engaging a Pharma Audience All Year Round
For pharma manufacturers and service providers, attending face-to-face events and exhibitions has always been a crucial tool for meeting existing clients, signing contracts, and gathering leads for new business. But with events unable to trade over the... -
News CPHI Webinar: How is M&A Shifting the Pharma Manufacturing Landscape?
Watch this CPHI Webinar sponsored by PharmaVentures, in which a broad panel of experts across the pharmaceutical manufacturing space discussed how M&A activity is being shaped by this ever evolving and complex sector -
News Recipharm selected to operate new fill/finish facility in Morocco
Move is part of US$500 million five-year investment programme by Moroccan government and consortium to establish vaccine and biotherapeutic manufacturing capacity in the country -
News Second Curaleaf GMP cannabis facility gains Spanish approval
Medalchemy facility now fully licensed to import, cultivate, extract, manufacture and export medical cannabis flower and extracts -
News Viralgen opens expanded AAV manufacturing facility in Spain
The CDMO's fivefold capacity increase will provide early preclinical through to commercialisation support -
News Amgen to build new assembly and final packaging facility in Ohio
Greenfield construction of the new facility is expected to start later this year
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance