All News
All news
-
News CPHI Worldwide Advisory Board returns with five new global experts
Board helps to steer the overall development of CPHI events and content. -
News Optibrium and Goldfinch Bio sign agreement for StarDrop license
Goldfinch Bio’s researchers select StarDrop to guide successful drug discovery. -
News Catalent provides commercial manufacturing of Lexicon Pharmaceuticals' orphan drug Xermelot following FDA approval
Xermelo is a first-in-class orally administered therapy for the treatment of carcinoid syndrome diarrhea. -
News Bosch Packaging Technology presents Xelum platform for continuous manufacturing
Highly precise dosing of smallest amounts of API. -
News New buprenorphine wafers could help thousands battling opioid addiction
When compared with current hard sublingual tablets, the novel formulation dissolved quickly with no increased safety concerns and no demonstrable difference in efficacy. -
News Cross-industry partnership reduces the cost of popular diabetes, asthma and other brand name drugs
Inside Rx, a new initiative from Express Scripts, partners with GoodRx, drug manufacturers and drug stores to save Americans an average of 34% at 40,000 pharmacies -
News First approval for Keytruda in a hematologic malignancy in the EU
European Commission approves Keytruda for patients with relapsed or refractory classical Hodgkin lymphoma who failed ASCT and BV or who are transplant-ineligible and have failed BV. -
News Cherwell Laboratories to highlight cleanroom microbiology solutions at The Clinical Pharmacy Congress
A key focus on Cherwell’s Stand A49 will be the microbiology requirements for pharmacy aseptic manufacturing, preparation and dispensing. -
News ProSolus doubles transdermal drug delivery manufacturing capacity
New ProSolus commercial team will provide potential partners throughout the pharmaceutical industry direct access to the state-of-the-art research, development, and manufacturing capabilities. -
News SGS Clinical Research announces results of FiH studies using novel virus as experimental challenge agent
Strain represents one of the common current circulating strains of influenza of a pandemic origin. -
News Arch Biopartners starts GMP manufacturing for AB569 at Dalton Pharma Services
Company will be responsible for the GMP preparation and filling of AB569 into glass vials. -
News Sartorius Stedim Biotech launches chemistry testing services
This chemistry testing offer complements the company’s existing range of off-the-shelf biological assays for biosimilar products, providing a complete solution from one contract partner. -
News SGS Announces Results of First-In-Human Studies Using Novel Virus as Experimental Challenge Agent
SGS announced the successful completion of a Phase I clinical trial of a new strain of influenza virus in healthy volunteers. -
News Aptar Pharma partners with Kali Care to develop real-time medication management technology
Combined expertise is likely to have a significant impact in reducing the costs and complexity of ophthalmic clinical trials. -
News NIH discovery in mice could lead to new class of medications to fight mid-life obesity
With lower rates of obesity, the researchers say rates of heart disease, diabetes, and other diseases that tend to increase with age, including cancer and Alzheimer’s disease, could fall as well. -
News Isogenica introduces next-generation, fully synthetic human Fab antibody fragment library
Library claimed to offer "the most efficient route to therapeutic antibody lead identification". -
News Native Antigen Company expands into new development and production facilities
Expansion doubles manufacturing capacity and includes the introduction of new facilities. -
News AstraZeneca’s Imfinzi receives FDA accelerated approval for previously treated patients with advanced bladder cancer
Imfinzi is the cornerstone in an extensive immuno-oncology program across multiple cancer types and stages of disease. -
News Concert Pharmaceuticals initiates CTP-543 Phase II trial in alopecia areata
Trial designed to evaluate the safety and efficacy of CTP-543 after 12 months of dosing with the primary efficacy analysis at week 24. -
News GSK invests $139 million to expand production capacity for Benlysta in Rockville
Site is also expected to house production of a new subcutaneous form of belimumab, which is currently under review with the FDA. -
News Shire and Parion Sciences sign collaborative license agreement to advance P-321 for ophthalmic indications
P-321 is a Phase II investigational topical treatment for dry eye disease. -
News Impax announces FDA approval and launch of a generic version of Vytorin
One of the first companies to offer a generic version of Vytorin. -
News Takeda announces FDA Accelerated Approval of Alunbrig
Alunbrig approved for ALK+ metastatic non-small Cell lung cancer patients who have progressed on or are intolerant to crizotinib. -
News China FDA approves country’s first all-oral regimen for chronic hepatitis C, Daklinza in combination with Sunvepra
Daklinza and Sunvepra combination approved for genotype 1b, the most common chronic hepatitis C (HCV) genotype in China; combination has a 91-99% cure rate. -
News Sartorius introduces new BIOSTAT STR bioreactor range and new Flexsafe STR single-use bags
New generation of bioreactors combined with new Flexsafe STR bags offer a fully scalable, single-use system. -
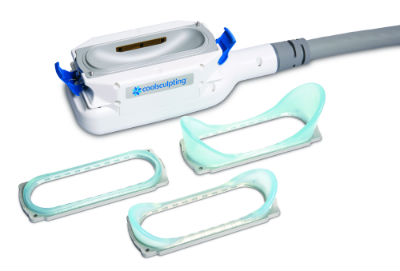
News Allergan successfully completes ZELTIQ Aesthetics acquisition
Acquisition adds best-in-class body contouring business to Allergan's facial aesthetics, plastic surgery and regenerative medicine businesses. -
News Record growth for life science tech firm as MES adoption increases globally
Zenith Technologies surpasses the €20 million milestone for sales of its manufacturing execution system (MES) services. -
News Learn from change-making pharmaceutical leaders: CPHI North America keynote speakers announced
CPHI North America will feature the first-ever Connect Conference. -
News Antidepressant may enhance drug delivery to the brain
NIH rat study suggests amitriptyline temporarily inhibits the blood-brain barrier, allowing drugs to enter the brain. -
News I Holland offers free trial of its innovative tool management system
System allows tablet manufacturers to keep a record of tablet quantities by number of tablets, work order or batch information to ensure production is run efficiently. -
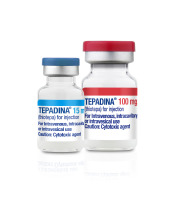
News First FDA-Approved 100 mg Thiotepa Now Available through Amneal Biosciences as TEPADINA® (thiotepa) for Injection
Thiotepa is now available through Amneal Biosciences in both 100 mg/vial and 15 mg/vial as branded TEPADINA® (thiotepa) for injection. The product is supplied as a powder for solution in single-dose vials for intravenous, intracavitary, or intravesical... -
News Pharmatek adds further cGMP spray drying capacity to meet demand for improved pharmaceutical solubility
Company has also installed a high pressure, precision roller compactor to further support the development of spray-dried dispersions. -
News Shire granted EU conditional marketing authorisation for Natpar for the treatment of chronic hypoparathyroidism
Natpar is the first and only licensed recombinant human parathyroid hormone therapy for chronic hypoparathyroidism. -
News Fresenius Kabi to strengthen and diversify product portfolio by acquiring Akorn and Merck KGaA’s biosimilars business
Transactions provide access to attractive pharmaceutical growth markets. -
News Regeneron and Sanofi receive FDA approval of a new once-monthly dosing option for Praluent Injection
Monthly dosing schedule now approved in both US and EU. -
News A new lipid technology for antiseptic emollient products
Formulations can be delivered as spray, drop, solution, medical wipe or dermal patch.and offer non-touch applications. -
News Novartis expands global collaboration with Amgen to commercialize first-in-class AMG 334 program in migraine prevention
Novartis and Amgen to co-commercialize AMG 334 (erenumab) in the US; Novartis to gain exclusive rights in Canada, -
News Sartorius group invests $100 million in expansion of its plant in Yauco
Is the largest single investment Sartorius Group has made outside Goettingen in the current year. -
News Allergan granted marketing authorization by FDA for TrueTear
The first intranasal neurostimulating device proven to temporarily increase tear production. -
News Marken Launches 24 Hour Patient Call Center
Marken has launched a 24 hour, 7 day a week call center for patients enrolled in clinical trials. The call center, known as Marken’s Patient Communication Center, is based in Philadelphia (USA) and is dedicated to the logistics needs of patients who pa... -
News Web monitoring for capturing real-world feedback on drugs
First live demo of PLG’s powerful new AI-based social media listening solution, PLG inVolv, designed to help life sciences firms meet their pharmacovigilance obligations. -
News Sanner passes 60 million euro mark
Significant investments in Bensheim headquarters; consistent portfolio additions; cleanroom expansion in China. -
News Recipharm opens new GMP suite for clinical trial material
The $750k investment is intended to produce CTM for clinical studies up to Phase II for non-sterile dosage forms, including metered dose inhalers and semi-solid topical products. -
News Lilly's breast cancer study of abemaciclib demonstrated superior progression-free survival at interim analysis
The Phase III study compared abemaciclib in combination with an aromatase inhibitor versus an aromatase inhibitor alone in patients with HR+, HER2- advanced breast cancer. -
News Recipharm delivers first serialised batch to Saudi Arabia
Company has invested €300,000 into the project, which is part of a wider €40 million investment into new serialisation technology and processes to comply with the European Falsified Medicines Directive. -
News Sandoz proposed biosimilars rituximab and etanercept recommended for approval in Europe
Company receives positive CHMP opinions for biosimilars rituximab and etanercept to treat immunological diseases. Biosimilar rituximab also recommended to treat blood cancers. -
News BMS-986036 shows consistent improvement in liver fat, liver injury and fibrosis in patients with NASH in Phase II trial
Primary endpoint of significant reduction in liver fat achieved following 16 weeks of treatment with BMS-986036. -
News Pfizer unveils ATLAS, an Interactive, website that provides global antibiotic resistance surveillance data across 60 countries
Website can help inform global health strategies to mitigate the threat of antimicrobial resistance. -
News CanniMed Therapeutics commences cannabis oils facility expansion
The GMP-compliant ethanol extraction facility will ensure ample supply of cannabinoid-based raw materials for future product development and innovation. -
News World’s largest society for regulatory professionals increases investment in Europe
An investment of more than 2 million euro over 3 years is planned to launch new events and resources. -
News Lilly to present data for galcanezumab for the prevention of migraine at the AAN annual meeting
Treatment could help to reduce the number of days lost to migraine. -
News BioInvent expands manufacturing facility with Mobius bioreactors from Merck
Fully scalable system streamlines drug development and production. -
News Porvair acquires J.G. Finneran Associates
Finneran bringing glass, plastics and assembly manufacturing capabilities and strong US distribution. -
News Merck partners with Drugs for Neglected Diseases initiative
Merck is opening its compound library to DNDi to help find cures for leishmaniasis and Chagas disease. -
News Nivalis Therapeutics and Alpine Immune Sciences agree to merge
Combined company well capitalized with $90 million in funding to advance discovery and development. -
News Fujifilm increases production capacity and establishes new process development facilities
$130M USD investment to support growing market demand. -
News Cardinal Health to acquire leading patient product portfolio from Medtronic for $6.1 billion
Increases Cardinal Health's product breadth in consumable medical products. -
News Cobra Biologics embarks on £15 m gene therapy manufacturing operations expansion
Capacity increase in response to customer demand for DNA and viral vector production. -
News FDA approves Genentech’s Lucentis for diabetic retinopathy
First and only medicine FDA-approved to treat all forms of diabetic retinopathy. -
News Allergan expands leading R&D NASH Program with Novartis Clinical Collaboration
Collaboration focused on Phase 2II clinical trial to evaluate use of Allergan's Cenicriviroc and Novartis' lead FXR agonist to treat NASH. -
News Cobra Biologics Embarks On £15m (165m SEK) Gene Therapy Manufacturing Operations Expansion
Over the next two years Cobra will invest up to £15m (165m SEK) on a phased expansion plan, supporting the company’s R&D expertise in developing rapid and cost effective viral vector and DNA plasmid production platforms. -
News FDA issues complete response letter for baricitinib
Additional clinical data are needed to determine the most appropriate doses. -
News BMS to license anti-eTau and anti-myostatic compounds
Two separate agreements with Biogen and Roche. -
News Scale Up in the pharmaceutical Industry
In the present application from the pharmaceutical process development, the aim is to transfer processes from the 70ml reactor into the 25lt scale, as efficiently and reproducibly as possible. In most cases, such a scale-up step takes place in differen... -
News Arch Biopartners' lead anti-bacterial drug candidate to enter investigator-sponsored Phase I human trial
AB569, which consists of two active ingredients, sodium nitrite and EDTA, has a novel mechanism of action that differs from that of antibiotics. -
News Regen BioPharma sees success in Its pre-clinical small molecule optimization program for NR2F6
The company's small molecule drug program is aimed at treating cancer and autoimmune diseases . -
News BMS and Apexigen collaborate to evaluate Opdivo in combination with APX005M in advanced solid tumours
Study to evaluate potential of APX005M plus Opdivo to activate antigen-presenting cells in the tumour micro-environment to demonstrate enhanced anti-tumour activity. -
News Takeda enters into strategic collaboration with NuBiyota for microbiome therapeutics
Collaboration to advance oral microbial consortia products developed by using NuBiyota’s microbiome platform for GI indications. -
News Lhasa and Optibrium enter research and product development collaboration
Drug discovery software specialists combine expertise to extend studies into drug metabolism modelling. -
News Amneal Introduces Its First Nasal Spray: Generic Nasonex®
Amneal Pharmaceuticals LLC has launched mometasone furoate nasal spray, the company’s first pharmaceutical product in spray form. Mometasone furoate, available in 50 mcg/spray strength, is an AB-rated therapeutic equivalent to Nasonex®.
-
News Colorcon launches Colorcon Academy
Industry leading further education programs unified and expanded. -
News Phase III study shows Genentech’s Alecensa was superior to crizotinib in a specific type of lung cancer
Results showed that people treated with Alecensa lived significantly longer without their disease progressing compared to crizotinib when given as initial (first-line) treatment. -
News BMS earns ENERGY STAR Partner of the Year Award for third consecutive year
Company has achieved an annual energy intensity improvement of more than 3% globally, contributing to an 11.4% reduction in energy consumption since 2010. -
News New technology can detect tiny ovarian tumours
“Synthetic biomarkers” could be used to diagnose ovarian cancer months earlier than now possible. -
News The connected factory - a reality for the process and packaging industry
Bosch showcases i4.0 solutions live at Interpack. -
News Allergan reports topline Phase II data supporting advancement of Botox for the treatment of MDD
Botox is currently being studied as a potential treatment option for adult patients with moderate to severe MDD. -
News FDA approves new indications for Harvoni and Sovaldi
First HCV direct-acting antivirals approved for use in adolescents. -
News Merck receives CRL from the FDA for TECOS study with sitagliptin
CRL relates to Merck's sNDA for Januvia, Janumet and Janumet XR. -
News Hikma enters into settlement agreement with Jazz for sodium oxybate
Agreement terms are "favourable for both parties". -
News Novartis to strengthen R&D pipeline by in-licensing ECF843 for ophthalmic indications
ECF843 is a new therapeutic approach and potential first-in-class Rx treatment in dry-eye. -
News RedHill Biopharma signs exclusive US license from Entera health for commercial GI product EnteraGam
RedHill expects to initiate US promotion of its two commercially-available gastrointestinal specialty products, Donnatal and EnteraGam, in mid-2017. -
News Millipore Express high area filters ideal for processing feed streams with high particulate levels
Greater filtration capacity with a smaller footprint than conventional filters, improving economics for biopharmaceutical manufacturing. -
News Vectura partner launches Breelib nebuliser in Poland
First launch of product using FOX smart nebuliser. -
News Kite presents promising preclinical data from KITE-585, a fully human anti-BCMA CAR T-cell product candidate
Phase I clinical study of KITE-585 in patients with multiple myeloma planned for 2017. -
News Sanofi appoints Bill Sibold Executive Vice President Sanofi Genzyme and Member of the Executive Committee
David Meeker will leave the company at the end of June after a 23-year career with Genzyme and Sanofi. -
News GS1 standards are vital for pharma serialization
Earlier this month, Verify Brand contributed a byline to Pharmaceutical Commerce about the importance of standards. We recently announced that Verify Brand made platform enhancements to the existing functionality of our software’s G... -
News GS1 standards are vital for pharma serialization
Earlier this month, Verify Brand contributed a byline to Pharmaceutical Commerce about the importance of standards. We recently announced that Verify Brand made platform enhancements to the existing functionality of our software’s G... -
News Catalent expands Kansas City clinical storage and secondary packaging capabilities
The five-fold increase in capacity reflects growing demand for cold chain services. -
News Innova Biosciences introduces Biotin Check&Go! Kit
Kit for confirming successful antibody-biotin conjugation. -
News Amgen submits applications in the US and Europe to expand current indication for Xgeva
Applications include data from the largest international trial conducted in multiple myeloma. -
News FDA now happy with Alkem's API facility
Company's CAPA addresses the three Form 483 observations. -
News Sharp announces £9 million investment
Will fund a new manufacturing, packaging and distribution facility. -
News Novartis drug combination Tafinlar + Mekinist receives EU approval for BRAF V600-positive advanced NSCLC
New indication of Tafinlar and Mekinist in advanced NSCLC provides only therapy approved in the EU for BRAF V600-positive NSCLC. -
News Launch of Utibron Neohaler in the US
Represents the latest combination therapy now available for the millions of patients living with COPD in the US. -
News Bruker introduces novel NMR solutions at ENC 2017
New magnetic resonance technologies and applications open new frontiers for biological, chemical and materials research and routine analysis in pharmaceutical and chemical industries. -
News TraceLink announces NEXUS 17 keynote speakers
Speakers to include leaders from Santen, EMVO, Recipharm, MIT. -
News Mylan expands voluntary recall of EpiPen
Recall will extend to additional markets in Europe, Asia, North and South America. -
News BMS and Incyte to advance the combination of Opdivo and Epacadostat into first-line registrational trials
Companies to initiate Phase III registrational trials in first-line NSCLC across the spectrum of PD-L1 expression and first-line head and neck cancer in 2017. -
News PCI Pharma Services Announces Significant expansion in Serialization technology
We are pleased to announce a significant expansion of our market leading Serialization capability. We will increase Serialization capacity across our global supply network to support clients in advance of meeting both US DSCSA and EU FMD implementation... -
News Additional Cryogenic Storage Capacity at Rockford, IL Facility
PCI Clinical Services (PCI) are pleased to announce a second phase 400 percent increase in Cyrogenic storage capabilities at our Rockford, Illinois site. The announcement follows our initial capital investment in specialist cryogenic storage in Rockfor... -
News Capsugel expands late-stage inhalation product development capabilities
Installation of state-of-the-art Harro Hӧfliger encapsulation unit expands clinical- and commercial-scale capacity for dry powder inhalation applications. -
News Cherwell makes an impact with new continuous monitoring microbial air samplers
ImpactAir high performance air sampler range joins Cherwell’s portfolio of cleanroom microbiology products. -
News Bosch launches new KLV series for container closure integrity testing
Inspection of up to 600 glass containers per minute using unique group testing. -
News Mylan completes acquisition of Cold-EEZE brand
Cold-EEZE is now Mylan's largest US consumer healthcare brand. -
News FDA approves Genentech’s Ocrevus for relapsing and primary progressive forms of MS
An important new treatment option for people with relapsing forms of multiple sclerosis demonstrating superior efficacy on the three major markers of disease activity compared with Rebif. -
News Tesaro announces FDA approval of Zejula for women with recurrent ovarian cancer
US commercial launch planned for late April. -
News Merck introduces Mobius MyWay portfolio for customized single-use assemblies
Offers more flexibility, better supply predictability and shorter lead times for more efficient and safer drug manufacture. -
News Analytical protein aggregate separation without a column
System can achieve separations with higher resolution, reproducibility and recovery then has previously been possible. -
News Chemotherapy as a tablet instead of an intravenous infusion
New production method for solid dispersions of docetaxel and paclitaxel. -
News Regeneron and Sanofi announce FDA approval of Dupixent
First targeted biologic therapy for adults with moderate-to-severe atopic dermatitis. -
News Encouraging results of Phase IIa study with Evenamide in patients with schizophrenia
Unique mechanism: glutamate modulation and voltage-gated sodium channel blockade. -
News Eprex marketing authorisation extended to include treatment of symptomatic anaemia
French Health Authority ANSM grants approval in the Mutual Recognition Procedure; the relevant health authorities are required to implement the new indication within 30 days. -
News Pfizer receives positive CHMP opinion for Trumenba for prevention of meningococcal group B disease
Trumenba has been studied in a global clinical development program evaluating the vaccine in adolescents and adults. -
News Invivotek completes solar farm and facility expansion
Provides a cleaner, renewable energy source to reduce costs and lessen the CRO facility's impact on the environment. -
News BMS receives positive CHMP opinion recommending Opdivo for head and neck cancer
Opdivo already approved by the EC for six indications in four distinct tumour types. -
News FDA approves Parkinson's disease drug
First NCE approved for PD patients with motor fluctuations in the US in more than a decade .
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance