All News
All news
-
News Fujifilm Diosynth Biotechnologies to expand biologics manufacturing capacity
As part of $850 million investment package, CDMO will double US cell culture production for recombinant vaccines and increase UK gene therapy production tenfold -
News WuXi STA to build second US pharma manufacturing site at Middletown
Facility is expected to come online in 2024 and will feature testing laboratories, manufacture of active pharmaceutical ingredients and manufacture and packaging of solid dosage pharmaceuticals and sterile products -
News Sanofi to accelerate mRNA vaccines through new Center of Excellence
The company will invest €400 million per year to advance mRNA technology beyond the pandemic -
News Lonza to invest in mid-scale API manufacturing expansion at Chinese facility
The expansion of mid-scale capacity will enable the company to offer a smooth transition between early-phase and large-scale commercial production -
News CPHI Podcast Series: M&A strategies in the CDMO sector
This month we discuss what is driving M&A strategies in the pharma space, paying particular attention to how investors regard the potential for the growing but fragmented CDMO sector -
News US Pharma Market 2022 and Beyond - Download Report Now!
Ahead of the return of CPHI North America, Informa Markets has commissioned an in-depth report into the US pharma market focusing on how the pandemic has affected public discourse and political priorities, and how it could alter the pharmaceutical supp... -
News Genezen begins construction of cGMP lentiviral production facility
The investment will provide capacity to support current and future demands for the rapidly evolving cell and gene therapy sector -
News CPHI Webinar: Trends and Advances in Patient Centric Drug Development - Watch On Demand
Catch up on the latest trends and developments in patient centric drug development -
News Vectura to provide preclinical support for Incannex's inhaled brain injury treatment
IHL-216A may make sports safer and reduce the morbidity and mortality rates of people suffering serious head traumas -
News ProBioGen and LAVA Therapeutics sign cell line development agreement
The agreement marks the second cell line development collaboration between the two companies -
News Coriolis Pharma expands facilities for ATMP development
The expansion close to company's Martinsried headquarters includes a lyophilisation development centre for advanced therapy medicinal products -
News Cannabinoids: The Next Frontier for Cosmeceuticals?
The market for CBD is growing and it is not just the pharmaceutical industry that is exploring its potential. Martin Oxley, MD at buzzback UK explains how consumer interest in plant-based ingredients and natural products present huge opportunities for ... -
News Monosodium Phosphate 2-hydrate is now available with CEP
Dr. Paul Lohmann® recently received a CEP (Certificate of Suitability to the European Pharmacopoeia) for Monosodium Phosphate 2-hydrate, which enables a customer to register a drug in several countries in a cost-saving and simplified way. Since thi... -
News Pharmapack interviews Serkan Oray of UCB Pharma
Ahead of Pharmapack Europe 2021 in October, we talk to one of the speakers, Serkan Oray, Vice President, Devices, Packaging & Wearable Technologies at UCB Pharma about the company's new electromechanical injection device and the innovation drive wi... -
News Pandemic creates extra demand for CDMO services but investment decisions remain key to success
While the COVID-19 pandemic has created even greater demand for partnerships, CDMOs need to be aware that the needle has shifted, creating both opportunities and challenges and changing how the services industry does business -
News CPHI Trend Report - Sustainability in Pharma
While in the past, much of the discussion around sustainability in the pharmaceutical industry rightly focused on efforts to minimize the environmental impact of drug production, the needle has now shifted. There are signs that companies not only under... -
News Bayer makes €250 million manufacturing investment in Finland
The company expects to complete its building and modernising plans in Turku by 2025 -
News Robust sales growth at Indian pharma companies expected in current FY on pandemic recovery, says Fitch
Drug categories impacted by travel restrictions expected to see higher sales levels in FY22 -
News Construction underway for Yposkesi's second commercial bioproduction site
The French CDMO is also creating a new global resource for drug developers of ATMPs -
News Continuous manufacturing: CDMOs have a huge role to play in helping deliver wider adoption
During CPHI Discover (17th-28th May, 2021) – global pharma’s largest ever virtual gathering – we spoke with Doug Hausner, Senior Manager Continuous Manufacturing at Thermo Fisher Scientific. He presented his views on how continuous ma... -
News WuXi XDC and LegoChem sign MoD to make more ADCs accessible to patients
XDC will provide a full range of development and manufacturing services in one centralised region -
News First-of-its-kind Alzheimer’s drug gains US FDA approval
Biogen and Eisai's Aduhelm approved using the accelerated approval pathway after clinical trials demonstrated it reduced amyloid beta plaques by up to 71% -
News Entegris to expand 3 life sciences manufacturing facilities
The expanded capacity and capabilities include the development of a dedicated Life Sciences Technology Center -
News EmpowerPharm certified to manufacture CBD products to prescription drug standards in Canadian first
New $30 million manufacturing facility in Burlington, Ontario, will allow company to manage R&D in-house and scale production without reliance on third-party manufacturers -
News Pandemic inevitably slows pharma M&A activity but appetite for deals remains
The initial outbreak of the COVID-19 pandemic delayed merger and acquisitions in the pharma sector, but evidence points to an uptick in activity because the rationale for sealing deals hasn’t changed -
News The Top Ten CPHI Discover Company Showcases
CPHI Discover (May 17-28) gave Pharma companies, suppliers and service providers the opportunity to keep potential buyers and partners abreast of the latest news, product information and market trends in the form of free-to-access, downloadable content... -
News Pharma supply chain companies form alliance to accelerate sustainability
Alliance to Zero aims to facilitate the transition of the pharma sector to reach net zero emissions target -
News Lonza to install new production line at Geleen to increase Moderna COVID-19 vaccine output by 300 million doses per year
Drug substance production line expected to be operational by end of 2021 as part of CDMO’s expanded collaboration with biotech -
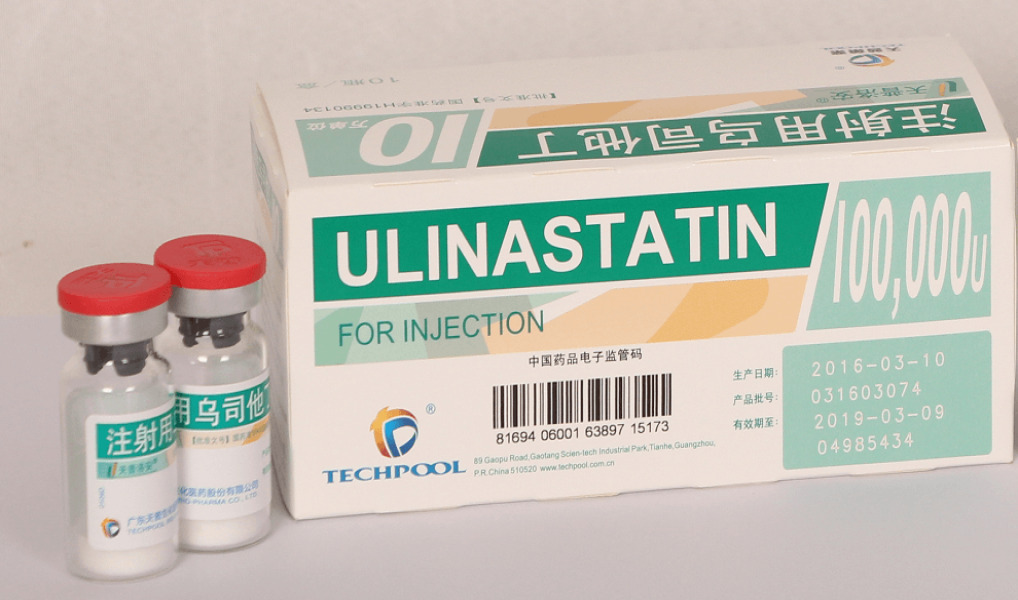
News Techpool UTI Blocks Cytokine Storm & Fights Against COVID-19
Cytokine storm is a main cause of worsening and death of COVID-19 patients. Techpool UTI inhibits the release of Inflammatory Cytokines and blocks Cytokine Storm in time to treat COVID-19 patients. -
News Siegfried starts to ramp up production after cyber attack
Hameln plant which provides fill and finish for Pfizer-BioNTech COVID-19 shot should be fully operational within the week, CDMO says -
News MedPharm expands topical and transdermal delivery services
A new location in Raleigh-Durham, North Carolina, close to the CDMO's Center of Excellence, will double its current footprint -
News Bacthera secures double nod to tread on "virgin territory"
The specialist CDMO receives manufacturing licenses from Switzerland and Denmark to supply customers with live biotherapeutic medicines -
News COVID-19 Pharma Development Tracker
The latest coronavirus updates and developments impacting the global pharmaceutical supply chain (continued from here) -
News Merck KGaA boosts lipid capacity to meet vaccine demand
The company's new synthetic cholesterol product will help meet the demand for mRNA vaccines, including Pfizer-BioNTech Covid-19 Vaccine -
News Sharp makes land acquisition to expand cGMP packaging capacity
The purchase is part of $17 million capacity expansion, which is in addition to a wider $43 million investment into the company's Pennsylvania operations -
News Finding the right experts and collaborating at speed essential to COVID-19 vaccine rollout, says CPHI panel
CPHI Discover webinar examines the progress made so far in terms of vaccine distribution to the global population as well as anticipating future challenges -
News API market tested by COVID-19 but pandemic is accelerating adoption of supply chain solutions: CPHI panel
CPHI Discover panel of industry experts discusses impact of COVID-19 on API manufacturing and trading as well as the controversial topic of reshoring production -
News ‘Conscious consumer’ demand is propelling cosmeceuticals industry, experts tell CPHI Discover audience
Upstream raw materials suppliers seen as the main source of innovation in a growing industry driven by demand for more natural ingredients manufactured in clean, sustainable way -
News UPDATED: The CPHI Discover Blog
Bookmark this page for regular updates on the rich variety of content coming your way via CPHI Discover, pharma’s largest ever virtual gathering, over the course of our three-day agenda -
News Pharmapack Podcast Series: Senior patient-friendly drug device design - Part Three
Partnering with one of northern Europe’s oldest academic universities, Lund University in Sweden, Pharmapack has launched this three-part podcast series, delivering the latest insights on medication packaging innovation and the gathering momentum... -
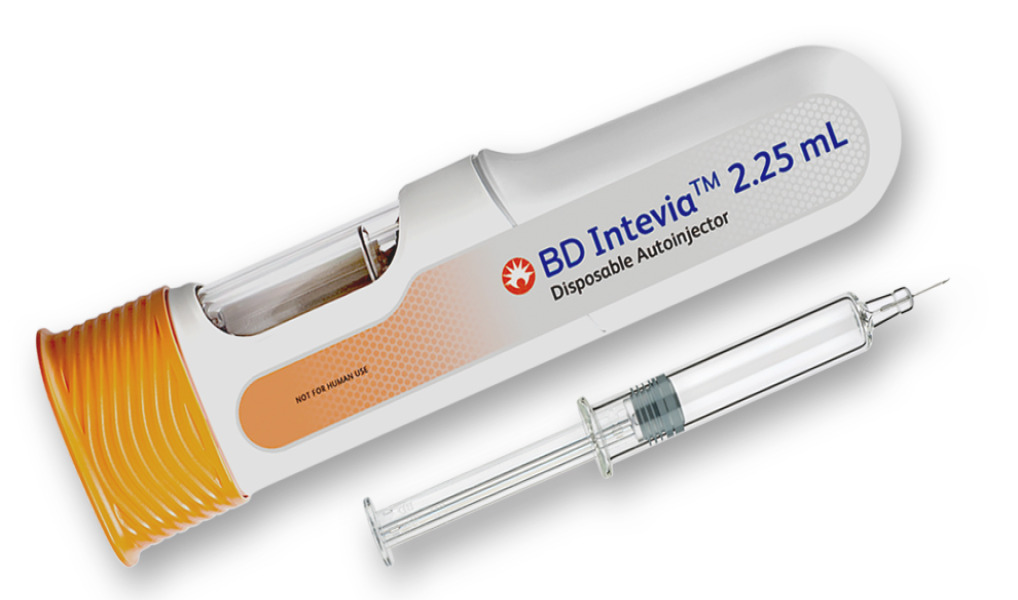
News Innovating in the viscosity design space with a new 2.25 mL autoinjector platform
Extending its patient-centric BD Intevia™ Disposable Autoinjector platform, BD builds on past successes while meeting the requirements of new, highly viscous, high-volume biologics for chronic diseases -
News Self-administration trend driving demand for subcutaneous drug delivery devices, says BD expert
Dosing complexity means programmable devices need to address several requirements around wearability and flexibility, CPHI Discover delegates are told -
News Overall benefits of ready-to-administer systems outweigh the initial cost disadvantages, says BD expert
Pre-fillable syringes for intravenous drugs can replace traditional glass vials and ampoules, particularly in the time-sensitive and fast-paced world of emergency care -
News COVID-19 has exacerbated contract manufacturing challenges but industry continues to respond well to crisis: experts
Coronavirus pandemic has exacerbated manufacturing capacity constraints, particularly in injectable dose and viral vector production, CPHI audience told -
News Drug delivery start-ups recognize benefits of contract manufacturing: CPHI panel
Strong and effective partnerships with CDMOs are essential to success due to the complexity of manufacturing processes -
News Drug repurposing can shorten drug development timelines, experts tell CPHI Discover audience
Process of identifying new uses for approved drugs can be done through different pathways and can yield some surprising benefits -
News Brexit impact on UK pharma industry a moveable feast, industry experts tell CPHI Discover
The UK’s withdrawal from the European Union has meant the pharmaceutical industry has had to adapt to some monumental changes in terms of research, regulatory affairs, and trade. In this CPHI Webinar, three industry experts discussed what the fut... -
News BD chooses Spain as home for new high-tech manufacturing facility
Zaragoza plant will produce devices such as prefillable syringes to deliver vaccines and other biologics -
News Europe approves SK Bioscience’s global COVID-19 vaccine plant
CDMO's site becomes the first vaccine production facility in South Korea to obtain EU GMP certification, allowing it to ship to Europe -
News APIs, excipients and formulation: the bedrock of medical innovation
Our virtual CPHI Discover event (17th-28th May) is just around the corner, full to the brim with three main content tracks. Here, we explore the major trends in APIs, Ingredients & Formulation -
News Beyond the box: Device and packaging tech innovation vital for pharma
Our virtual CPHI Discover event (17th-28th May) is just around the corner, full to the brim with three main content tracks. Here, we explore the major trends in Drug Delivery and Packaging. -
News Sterling Pharma Solutions signs up to manufacture OncoTEX's ovarian cancer drug
The CDMO's expertise and capabilities a "perfect fit" for overcoming the challenges of manufacturing this complex molecule -
News Drug R&D innovation reshaping manufacturing and the CDMO sector
Our virtual CPHI Discover event (17th-28th May) is just around the corner, full to the brim with three main content tracks. Here, we explore the major trends and issues surrounding Manufacturing and Outsourcing. -
News Catalent buys Belgian CDMO to boost pDNA manufacturing and service offering
New facility at Gosselies site will host commercial scale pDNA manufacturing as Catalent extends its cell and gene therapy footprint -
News AGC Pharma Chemicals Europe invites you to a live guided virtual tour
Step inside our state-of-the-art plant to learn about our small molecules CDMO services and meet our team with a virtual tour that focuses on your areas of interest. -
News CPHI Trend Report: The Future of Indian API Manufacturing - Out Now!
Read this new CPHI Trend Report which, via interviews with key industry experts, explores the state of India's API manufacturing issue and how it is being impacted by the country's ambitious healthcare reform programme as well as efforts by the governm... -
News CPHI Discover: Earlier partnerships between pharma and excipient manufacturers are the key to success
In the future pharma companies will need to work closer with excipient companies if we are to develop better formulations more quickly -
News Ex-Legacy Pharmaceuticals machinery and equipment up for sale, says Hilco Industrial
Industrial assets purchaser is offering high-capacity production line equipment formerly owned by bankrupt CMO -
News Moderna to transform Massachusetts site into industrial technology centre
The expansion will support a 50% increase in production of biotech's COVID-19 vaccine at its manufacturing site -
News CPHI Discover: How is the Biden Administration impacting the US pharma market?
Ahead of the session, How will the New Administration Impact the US Pharma Market? at CPHI Discover on Tuesday 18th May – global pharma’s largest ever virtual gathering – we spoke with one of the speakers, Nielsen Hobbs... -
News Clean Biologics buys CDMO Biodextris
Transaction enables ArchiMed-backed biopharmaceutical services group to establish North American presence and expand activities with complementary services -
News CPHI Discover: Remote excipient audits are on the increase, but we must do more to develop novel excipients
Ahead of the session, Value Added Excipients to Unlock the Potential of APIs, at CPHI Discover on Thursday 18th May – global pharma’s largest ever virtual gathering – we spoke with one of the speakers, Dr Iain Moor... -
News Podcast: CPHI Discover – A look ahead to three days of insightful content: Listen Now!
Our online pharma event CPHI Discover is almost upon us! While we’ll be delving into the typical themes of ingredients, manufacturing, supply chain logistics and technology, we’re also going to be examining some newer trends which are impac... -
News N-SIDE launches Production App to optimise clinical manufacturing process
The software consulting company claims its software solution has already saved one pharma company €10.5 million -
News Moderna signs agreement with Gavi to supply COVID-19 vaccine
Biotech ties up deal three days after World Health Organization issued an emergency use listing for the mRNA vaccine -
News CPHI Webinar Series: How to Drive Sustainable Packaging Development in a post-COVID world?
To remain competitive, pharma companies must now collaborate with suppliers on how their packaging processes address sustainability. This recent webinar available on demand provides an overview on the drive for sustainable development and improving the... -
News Inclusion is everything: expanding diversity in clinical trials
How is the pharmaceutical industry tackling the issue of ensuring minority populations are properly represented in clinical trials? -
News New Moderna agreement will double vaccine drug substance production at Visp, says Lonza
Three additional drug substance manufacturing lines will be added to existing ones at Swiss site and are expected to be operational in early 2022 -
News Pharma ramps up innovation in response to COVID-19
CPHI asks industry experts at Beximco Pharmaceuticals, Catalent, DIA and Thermo Fisher Scientific to weigh in on the remarkable innovations we have witnessed and what lasting impacts they may have on our industry in a post-pandemic world -
News Alibaba bans online listing and trade of pharmaceutical products
Sweeping ban includes APIs, intermediates and prescription and OTC medicines and comes amid tight regulatory requirements and the "high risk nature" of pharma products, e-commerce giant says -
News Bushu and Suzuken team up to support manufacturers eyeing up Japanese market
The new business alliance will support new product launches for specialty pharmaceutical manufacturers looking to enter the Japanese market -
News Sanofi to provide fill and finish manufacturing support for Moderna vaccine
French company is now providing manufacturing capacity to support three different COVID-19 vaccines -
News Lonza to build small molecule manufacturing complex at Visp site
CHF 200 million investment will include dedicated line for antibody-drug conjugate payload molecules and is scheduled to start operations in Q3 2023 -
News Building supply chain resiliency to ensure access to medicines
With CPHI North America fast approaching, we ask Karen Flynn (President, Biologics & Chief Commercial Officer, at Catalent), Hamilton Lenox (Senior Vice President of Business Development, Sales & Marketing, at LGM Pharma), and Mike Kleppinger, Chief Co... -
News The cost of bringing US pharma manufacturing home
What are the major implications of any drive to reshore US pharmaceutical manufacturing, given that current Asian domination is based on cost savings and capacity? We ask the experts... -
News FDA inspection report slams Emergent for quality and cleanliness breaches at Bayview facility
Company failed to investigate vaccine drug substance discrepancies thoroughly, says US regulator -
News US Pharma Market Trends 2021 - Download Report Now!
2020 will go down in history as a pivotal year for the US pharmaceutical manufacturing sector. Firstly, the emergence of the global coronavirus pandemic provided a seismic shock to the industry and forced major rethinking on several lines. And secondly... -
News J&J to resume rollout of COVID-19 vaccine in EU after positive EMA safety ruling
Agency’s safety committee concludes positive benefit-risk profile but orders warning about rare blood clots to be added to shot’s product information
-
News Bharat Biotech scales up Covaxin manufacturing capacity in India
The company will expand multiple facilities in Hyderabad and Bangalore to help reach its target of approximately 700 million doses per year of the COVID-19 vaccine -
News CPHI Discover: Two-Tier Contract Market in China as Top CDMOs Grab all the Growth
Ahead of CPHI Discover (17-28 May, 2021) - global pharma's largest ever virtual gathering - we spoke to CPHI Annual Report expert Vicky Xia, China Head at BioPlan Associates on the biologics trends she is seeing emerging in the contract services sp... -
News Sustainability drive also worth the investment for smaller pharma firms
Sustainability takes effort, but the benefits of economically viable, environmentally responsible operations are worth it, even for smaller drug companies -
News AGC Biologics and Rocket Pharma expand LVV manufacturing partnership
The expanded agreement will ensure Rocket Pharma's supply of lentiviral vectors across its pipeline -
News EU secures additional 100 million doses of Pfizer-BioNTech vaccine for 2021 delivery
Extra supply will bring total number of doses scheduled to be shipped to EU this year to 600 million -
News Sartorius expands manufacturing capacities in China and the UK
The expansions will enable the company's local customers to test equipment directly at the manufacturing site -
News Abzena to build US biologics manufacturing plant in North Carolina
New facility will be research partnering company’s sixth global site and will be dedicated to mammalian biologics -
News CPHI Japan open for business!
Informa Markets' first in-person trade show of the year welcomes attendees in Tokyo as face-to-face interaction recommences among pharma community -
News Let's Get Thermal!: Continuous Thermal Processing Webinar Series
See MTI Bioscience systems in action! MTI Bioscience is excited to demonstrate their Continuous Thermal Sterilizers live on Zoom®. Please attend the following free live events on May 5th and 12th at 11 am EST, hosted by Dr. John Miles, President of... -
News Lonza and Junshi Biosciences expand biologics manufacturing collaboration
The antibody-based products will be expressed using Lonza's GS Xceed Gene Expression System -
News Green chemistry: environmental and economic sense for pharma?
The concept of Green Chemistry first arose as a set of principles to minimize the environmental impact of chemical production but as one of its earliest adopters, the pharmaceutical industry has enjoyed several other benefits -
News Sanofi strengthens vaccines manufacturing capacity in Singapore
The company will invest €400 million in a new site to enable it to produce vaccines on a massive scale for Asia -
News KD Pharma acquires Switzerland-based cannabinoid manufacturer PhytoXtract
The global CMO plans to have a complete, licensed cGMP manufacturing facility online by the end of 2021 -
News Catalent boosts plasmid DNA manufacturing at Rockville with addition of ABEC fermenters
The company's custom single run single-use fermenters supplied on fast-track schedule to support flexible manufacturing of plasmid DNA -
News Bavarian Nordic receives $28 million Ebola vaccine order from J&J
The smallpox vaccine specialist will manufacture and deliver bulk drug substance of MVA-BN Filo vaccine during the second half of this year -
News Pope Scientific expands scope of offerings for Nutsche Filter-Dryer sizes, designs and systems
Increased range of sizes and available features, plus new options in ANFD systems and the popular Benchtop Nutsche Filter for scaleup and kilogram level production -
News CPHI Podcast Series: Pharmaceuticals in the Environment and Antimicrobial Resistance
Pharmaceuticals discarded in the environment pose a risk to fish and other wildlife, for example by affecting their ability to reproduce, by altering their behaviour in ways jeopardising their survival, or through direct toxic effects. Increased awaren... -
News Moderna and Catalent expand COVID vaccine manufacturing agreement
The extended collaboration comes after reaching the initial milestone of producing 100 million doses -
News Sterling Pharma seals acquisition of bioconjugation specialist ADC
By integrating its HPAPI capabilities with those at ADC Biotechnologies' Deeside facility, small molecule CDMO will be able to offer the development and manufacturing of toxin linkers -
News NextPharma completes purchase of Lonza lipid oral dosage form manufacturing sites
Ploermel and Edinburgh facilities employ around 390 permanent staff and will allow UK CDMO to expand its technology offering -
News CrystecPharma opens R&D facility in Haimen City, China
Facility will support UK-based crystal and particle engineering specialist's existing Chinese subsidiary to expand product development services -
News TCR2 bets on lead candidate with new cell therapy manufacturing facility
The US facility in Rockville is expected to accelerate the company’s commercial-scale manufacturing timelines of gavo-cel -
News Lonza to support Pionyr Immunotherapeutics' oncology drug development
The CDMO will provide cell line development, process development, drug substance and drug product manufacturing for Pionyr’s monoclonal antibody candidate -
News KD Pharma expands CDMO capabilities via asset acquisition
The former Rohner's manufacturing assets will enable omega-3 heavyweight to manufacture difficult, multi-step pharmaceutical intermediates and APIs -
News GSK to manufacture 60 million doses of Novavax vaccine for the UK
Pharma company will provide fill and finish manufacturing capacity at its Barnard Castle facility as early as the beginning of May 2021 -
News CPHI Webinar Series: Latest Approaches for Large Volume Drug Delivery
Innovation in the design and development of large volume drug delivery devices can solve the challenge of accommodating the increasing trend towards patient self-administration and home care while at the same time ensuring that drugs that require intra... -
News Nutrition additive supply chains 'nervous' as Suez blockage continues
Some seaborne deliveries are being rerouted or switched to air transportation as efforts to re-float stricken Ever Given container vessel move into fourth day -
News Origin Pharma Packaging opens new UK warehouse in significant capacity expansion
New site includes warehouse maagement system which packaging firm claims will increase picking accuracy and efficiency -
News The Evolution of Pharma R&D Models: Watch Webinar Now
While research and development (R&D) in the pharma sector has traditionally been largely constrained to in-house activity, companies are now much more open to the idea of outsourcing their innovation to remain competitive amid spiralling costs of drug ... -
News CURE Pharmaceutical gains DEA go-ahead to research psychedelics compounds
Company will use extension of Schedule I licence to start development of psychedelics-based pipeline to treat mental health disorders -
News China's Raybow Pharmaceuticals invests $15.8 million to expand US facility
The company will triple its capacity and workforce at its North Carolina site to increase its global capabilities and reach -
News The PSCI supports the Musi River Revitalization Initiative, Hyderabad India
A quarterly series of blog posts about responsible supply chain management from the PSCI Chair, Manjit Singh -
News Pharmapack Podcast Series: Patient-Centric Packaging Design & Innovation - Part Two
Partnering with one of northern Europe’s oldest academic universities, Lund University in Sweden, Pharmapack has launched this three-part podcast series, delivering the latest insights on medication packaging innovation and the gathering momentum... -
News Catalent signs ODT technology development agreement with Cybin
The CDMO's Zydis technology will help the Canadian biotech to develop fast-acting, shorter-duration formulations of its potential therapy for treatment-resistant psychiatric disorders -
News Fujifilm names North Carolina as location for $2 billion large-scale cell manufacturing site
CDMO subsidiary Fujifilm Diosynth Biotechnologies will operate the site which is expected to be operational in 2025 -
News Pharma Explained: Principles of Continuous Manufacturing
Pharma’s uptake of continuous manufacturing is slowly gaining traction as the technology does hold some key advantages over traditional batch manufacturing, such as the potential to realise cost savings and the promise of lower yield loss. -
News Pharma importers forced to reorganise supply chains due to Brexit complications, says medicines trade expert
EU/UK trade deal fell way short of UK’s initial expectations for medicinal products ahead of negotiations, says Peter Gough at NSF International -
News Pfizer to sell Chinese biologics manufacturing facility to WuXi
Multinational makes ‘difficult decision’ to halt presence in Chinese biosimilars market five years after $350 million investment -
News Wuxi Vaccines' Ireland site still on course for 2022 despite COVID challenges
Despite COVID-related challenges and supply chain issues, the CDMO's $240 million new facility is on target to open next year -
News Artificial intelligence a no-brainer for drug discovery
AI is helping pharma find the drug candidates with the greatest chance of success, according to experts who say therapeutic efficacy and cost reduction are the key dynamics -
News PCI Pharma Services expands Berlin cold chain clinical supply storage capabilities
The move ensures the service provider has the capacity to meet the needs of an evolving clinical market -
News Growing drug delivery CDMO Kindeva expands UK operations
US-headquartered company Kindeva seals deal for an R&D facility at Loughborough’s life sciences hub
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance