All News
All news
-
News Sartorius Stedim Biotech introduces Sartobind cassettes
New modular membrane chromatography solution for large-scale applications . -
News Biogen’s Imraldi, an adalimumab biosimilar candidate referencing Humira, granted positive opinion by CHMP
If approved, Imraldi will be the third anti-TNF biosimilar in Biogen’s portfolio in Europe. -
News Juniper Pharma Services brings PSD-1 spray dryer online
The addition enables the CDMO to scale-up early-phase bench top processes to support later stage clinical development and niche, low volume manufacturing requirements. -
News Aptar Pharma holds its first dermal drug delivery seminar in China
Attendees learnt how Aptar's solutions could be applied to the Chinese market in a changing regulatory environment. -
News FDA approves Mydayis – a once-daily option for ADHD symptom control in patients 13 years plus
Mydayis demonstrated improvements lasting up to 16 hours post-dose, beginning at 2 or 4 hours post-administration, compared with placebo, in total score on a skill-adjusted math test that measures attention in ADHD. -
News Arcinova investment reduces R&D and manufacturing time by more than a month
Arcinova has invested in a Uniqsis FlowSyn continuous flow reactor enabling them to develop and simplify the scale-up of a challenging API batch process. -
News Novartis achieves regulatory milestone for AMG 334 in migraine prevention with EMA filing acceptance
AMG 334 (erenumab) is the first anti-CGRP monoclonal antibody developed for migraine prevention to receive EMA regulatory filing acceptance. -
News Accelerating freeze drying cycle development with just seven vials
Compared with other small-scale R&D freeze dryers, the LyoCapsule lyophilizer is the only system that can monitor and independently control the wall temperature via use of the center vial product temperature. -
News The “BB Dose”: a Unither innovation that guarantees more safety with the infant
Recently, Unither Pharmaceuticals partnered with Biolane to developed a single-unit dose featuring a more rounded tip that fits perfectly with babys’ nostrils. The “BB dose” guarantees a greater safety of the gesture of the mother with the infant. ... -
News Takeda completes new manufacturing facilities at the Oranienburg site in Germany
The manufacture of solid dosage form pharmaceutical products would be transferred from the Osaka Plant to the new plant. -
News QuVa Pharma announces availability of sodium bicarbonate syringe
Compounded Sodium Bicarbonate PF 8.4% 50mEq per syringe is expected to help alleviate chronic drug shortage impacting the nation's hospitals. -
News Glenmark Pharmaceuticals licenses small molecule oncology compound from APC Therapeutics to expand IO portfolio
The compound has the potential to be used as a monotherapy or in combination with approved therapies to address unmet needs in cancer treatment. -
News Merck Ventures creates new immuno-oncology company iOnctura
iOnctura is set up to develop a pipeline of assets targeting immunosuppression in the tumour microenvironment. -
News FDA accepts Amgen's sBLA to expand indication for Xgeva to include multiple myeloma patients
Xgeva is indicated for the prevention of skeletal-related events in patients with bone metastases from solid tumours and is the number one prescribed agent by oncologists for this indication in the US. -
News J&J completes acquisition of Actelion
Actelion will now become part of the Janssen Pharmaceutical Companies of J&J. -
News Sandoz receives approval in Europe for Rixathon to treat blood cancers and immunological diseases
Sandoz now has four biosimilars approved in Europe - more than any other company. -
News FDA updates on Pfizer drug shortages
Pfizer says shortages caused by manufacturing, distribution and third party delays. -
News BMS to sell manufacturing facility in Swords, Ireland to SK Biotek
Company shifts manufacturing focus in Ireland to reflect growing biologics portfolio with ongoing investment in Cruiserath biologics facility. -
News Merck presses the pause button on Keytruda trials
The pause is to allow for additional information to be collected to better understand more reports of death in the Keytruda groups. -
News FlyPharma Conference: "Collaboration is the Key"
Delegates at the recent FlyPharma Conference pick out collaboration as their main goal for the coming year. -
News Parexel and Sanofi collaborate to advance the use of wearable devices in life science industry
Preliminary findings from pilot study validate use of wearable technologies to collect data and manage clinical trials more effectively and efficiently. -
News Lilly's Taltz shows promise for patients with active psoriatic arthritis
Demonstrated significant improvements in disease signs and symptoms at 24 weeks among patients with active psoriatic arthritis who had prior inadequate response or intolerance to TNF inhibitors. -
News LifeArc, previously MRC Technology, set to transform the medical research landscape
Investment will focus on new innovations in therapeutics for neurodegeneration, antibiotic resistance, cancer and respiratory diseases. -
News FDA accepts Sandoz regulatory submission for a generic version of Advair Diskus
Sandoz believes its combination product will offer asthma and COPD patients same safety and efficacy as reference medicine. -
News TraceLink expands European HQ to accommodate projected 207% EU employee growth in 2017
Extends EMEA sales team with addition of two new regional sales leaders in DACH and Southern Europe regions. -
News Orthogonal platform enhances protein separation and characterisation
Enables labs around the world to generate more useful information from complex proteins, antibodies, polypeptides and polysaccharides. -
News Irvine Scientific adds new Center of Excellence for R&D
Company adds fifth building in Southern California. -
News SGS expands extractables and leachables testing capabilities at it Shangahi facility
Investment allows company to increase its capacity to handle E&L projects for applications including container testing, medical device evaluation and testing equipment used in pharmaceutical production. -
News Successful Phase III top-line results with Bekinda for acute gastroenteritis
Study successfully met its primary endpoint and Bekinda 24 mg was shown to be effective, safe and well tolerated in patients with acute gastroenteritis and gastritis. -
News Achelios Therapeutics concludes successful Type C meeting with FDA regarding Topofen
Currently there are no approved topical NSAIDs indicated for the treatment of migraine. -
News Aker BioMarine Named Norway’s Most Innovative Business
Aker BioMarine, a biotech innovator and Antarctic krill harvesting company dedicated to improving human and planetary health, has been named as Norway’s most innovative company by Innovasjonsmagasinet, Norway’s leading innovation magazine. Innovasjonsm... -
News Lilly's galcanezumab significantly reduces number of migraine headache days
New results presented at AHS. -
News SGS successfully implements Oracle Argus solution for drug safety and regulatory compliance
Argus improves the quality and timeliness of safety case processing and reporting, and reduces operational risks for both clinical trial and post-marketed pharmacovigilance data. -
News Major boost for combatting drug addiction in Scotland as SMC gives green light to new wafer-thin treatment
Espranor represents the first major advance available in opioid substitution therapy for 10 years. -
News TraceLink unveils industry’s first Compliance and Digital Information Platform for pharmacies with EU FMD requirements
Application "addresses a critical information gap that has existed for decades between pharmaceutical companies, pharmacists and patients." -
News FDA requests removal of certain prescription opioid for risks related to abuse
Injection abuse of reformulated Opana ER has been associated with a serious outbreak of HIV and hepatit.is C -
News Aptar Pharma extends its child-resistant nasal pump manufacturing capabilities in North America
As of today, all major brands of nasal decongestant topical sprays in the US come with Aptar Pharma’s child-resistant feature. -
News Lubrizol invests $60 million to expand Particle Sciences, Vesta and other facilities
Investments will strengthen the excipients, polymers, drug formulation and manufacturing, and medical device contract manufacturing capabilities throughout the company's global facilities. -
News Bosch launches a pilot fermenter for manufacturing APIs
Highly flexible system for a broad range of processes. -
News Valeant sells iNova Pharmaceuticals for $930 million
Selling iNova part of Valeant's efforts to simplify and strengthen its balance sheet. -
News Lilly announces strategic collaboration with KeyBioscience
Collaboration to focus on a potential new class of treatments for metabolic disorders. -
News BIOCORP SIGNS AN INDUSTRIALIZATION CONTRACT WITH VIRBAC
BIOCORP signs an industrialization contract with Virbac for the manufacturing of an innovative administration and closure system compatible with vials. -
News FDA Advisory Committee to review Avastin biosimilar candidate
The Committee will review analytical, pharmacokinetic and clinical data from studies involving ABP 215, including results from a Phase III study in patients with NSCLC. -
News Merck's SOLu-Trypsin enzyme brings stability to mass spectrometry
Using the solution stable enzyme can eliminate unnecessary waste and cost. -
News Novatek International selected as partner for automation of Boehringer Ingelheim's global environmental monitoring program
NOVA-EM is Novatek's solution dedicated to managing all aspects of an automated environmental monitoring program. -
News TraceLink unveils Automated Validation Manager
Removes burden of validating software updates for Life Sciences Cloud customers. -
News New BMS collaboration to evaluate combination therapy in colorectal cancer
Phase I/II study to evaluate the combination of Opdivo (nivolumab) and Opdivo +Yervoy (ipilimumab) regimen with Mekinist (trametinib) in patients with colorectal cancer. -
News Pharmaceutical contract manufacturing/contract research market worth $238.3 billion by 2025
Expanding market for biosimilars and biobetters is anticipated to pronounce the demand for contract development and manufacturing support. -
News Lonza acquires cell and gene contract manufacturer PharmaCell
Lonza has successfully completed the acquisition of PharmaCell, a cell and gene contract manufacturer in Europe with employees in Maastricht and Geleen (NL). In 2016, PharmaCell had sales of EUR 11 million. -
News Acasti Pharma and CordenPharma announce large-scale production of CaPre with continuous manufacturing
Continuous manufacturing process will enable improved quality control and cGMP compliance, while reducing energy consumption, waste and raw material. -
News Colorcon expands in India
The new technical service laboratory is the company's 20th across the world. -
News Combination therapy shown to shrink tumours in many patients whose cancer has spread to the brain
Combination of nivolumab plus ipilimumab can fundamentally change survival expectations. -
News Sartorius Stedim Biotech launches ambr 15 bioreactor system with Nova BioProfile FLEX2 integration
QbD studies in upstream processing more rapidly performed. -
News Novartis announces ground-breaking collaboration with IBM Watson Health on outcomes-based care in advanced breast cancer
Collaboration will use real-world patient data and cognitive computing with aim of improving outcomes in advanced breast cancer. -
News Gen-Plus
Gen-Plus is a small medium-sized, privately owned company.
Its aim is to discover innovations for contract partners. We
design formulation and technology concepts for the
pharmaceutical industry. This includ... -
News Spinraza approved in the EU as first treatment for spinal muscular atrophy
Robust data from Phase III studies demonstrated positive impact on motor milestone achievement; increased survival in infants with SMA. -
News Extractables-free deep-well plate for uHPLC and MS applications
Porvair Glass Vial Deep-well plates are precisely manufactured to ensure complete compatibility with automated equipment. -
News NICE approves first use of Opdivo in blood cancer
NICE has recommended immunotherapy drug nivolumab for the treatment of adult patients with classical Hodgkin lymphoma. -
News Vertex reaches long-term reimbursement agreement with ROI for Orkambi, Kalydeco and future CF medicines
Agreement provides access to Orkambi for people who have two copies of the F508del mutation and expands access to Kalydeco for all eligible patients. -
News NIH researchers find potential genetic cause of Cushing syndrome
Finding may lead to therapies that prevent pituitary tumor recurrence. -
News Cardinal Health Specialty Solutions publishes research-based insights on oncology
Pharmaceutical companies should play a larger role in patient support programs. -
News Hikma signs an exclusive license and supply agreement with Octapharma for Octaplex
Deal adds the first plasma-derived treatment to Hima's product offering. -
News Sandoz proposed biosimilars adalimumab and infliximab accepted for regulatory review by the EMA
Biosimilar infliximab alone could potentially save the NHS £89 million. -
News Vetter and Microdermics to develop innovative microneedle drug delivery systems
Microdermics has successfully demonstrated the initial safety of its microneedle system, and is planning Phase I human clinical trials for vaccine and therapeutic delivery, to be initiated in 2017. -
News Synimmune initiates FiH study of Fc-optimized antibody Flysyn for the treatment of AML
Flysyn is the first antibody from the company's pipeline to be tested in humans. -
News Cambrex invests in new capacity and continuous flow technology at its Karlskoga, Sweden facility
The expansion is part of an ongoing strategic campaign to invest in small molecule API manufacturing across the company's global network of facilities. -
News Sanner acquisition strengthens company's leadership in effervescent packaging market
Acquisition of Jaco enables Sanner to operate as a system supplier. -
News Orchard Therapeutics signs manufacturing services agreement with PCT Cell Therapy Services
Under the terms of this new agreement, PCT will provide GMP-compliant manufacturing services for Orchard’s lead product, OTL-101, an autologous ex-vivo gene therapy for the treatment of ADA-SCID. -
News Hookipa Biotech announces data showing TheraT turns cold tumours hot
TheraT mediated alarmin release crucial for active immunization in cancer immunotherapy. -
News Recipharm makes strategic appointment in the UK & Ireland
Shabbir Mostafa to join the CDMO as Director, Business Management, with responsibility for driving sales of Recipharm’s manufacturing services. -
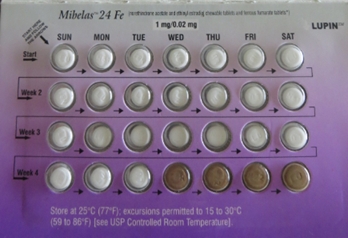
News Lupin Pharmaceuticals announces nationwide recall of Mibelas 24 Fe tablets
Recall due to out of sequence tablets and missing expiry/lot information. -
News Marken Launches Hybrid Clinical Trials Service
Marken today announced it has launched a new hybrid logistics service which leverages the UPS global transportation network, and has already received customer commitments for the new service. The hybrid service enables Marken to utilize the UPS network... -
News FDA accepts for priority review BMS’s application for Opdivo in previously treated hepatocellular carcinoma
Application is based on results from the Phase I/II CheckMate -040 trial. -
News Inovio HIV vaccine elicits nearly 100% immune response rates in a clinical study
Amongst highest levels of immune responses ever demonstrated in an HIV vaccine human study. -
News Pharmaceutical case studies illustrating data analytics strategies
New webinar hosted by Xtalks focuses on extracting the most value from data assets. -
News PDA initiative connecting drug manufacturers, glass suppliers to make manufacturing great
First initiative is to eliminate visible particles from parenteral products, -
News Innovation in packaging is here!!
Unipharma has applied state-of-the-art technology to traditional products and made the first ever Mono-Dose/Unit-Dose OTC line of products which will forever change the way people consume medications. No more confusions, no more charts, no more scie... -
News Merck strikes deal with Teijin Pharma for investigational antibody candidate targeting tau
The addition of this antibody targeting tau will complement Merck’s portfolio of candidates being investigated for the treatment of Alzheimer's disease. -
News WuXi STA, CFDA and Jinshan Government to co-host Marketing Authorization Holder regulatory summit in Shanghai
Industry and regulators combine to run afternoon congress on the implications of regulatory changes. -
News Hovione starts clinical trial of its proprietary minocycline sterile ointment to treat a subset of anterior ocular inflammation
First clinical study using a patent protected minocycline API and formulation developed by Hovione. -
News PharmaMar and Eczacıbaşı sign a licensing agreement for Aplidin in Turkey
Aplidin is PharmaMar's second most advanced anticancer drug currently under development for the treatment of multiple myeloma and angioimmunoblastic T-cell lymphoma. -
News IPF patients treated with Ofev vs placebo twice as likely to have improved or stable lung function
Separate subgroup analysis demonstrates long-term efficacy of Ofev in slowing disease progression is maintained for up to 96 weeks in IPF patients who require dose adjustments to manage adverse events. -
News Bioverativ to acquire clinical-stage rare disease biotechnology company, True North Therapeutics
Strengthens pipeline with lead candidate TNT009 in cold agglutinin disease, a rare and chronic autoimmune hemolytic anemia with no approved therapies. -
News OPKO R&D Facility
EirGen have commenced construction of a state of the art R&D facility in the Business and technology Park, Cork road, Waterford (Opposite the Whitfield clinic). The facility will be construction complete in Q2 2018 with a phased start up leading to... -
News New Rotogravure printing machine.
New rotogravure printing machine and a new cutting machine in operation since the end of 2014. -
News FDA approves Kevzara for the treatment of moderately to severely active RA in adults
Kevzara is now available to US patients. -
News New analyses reinforce the potential of Ultibro Breezhaler for COPD patients historically treated with steroids
New analyses from the FLAME study suggest dual bronchodilator Ultibro Breezhaler provides similar or better efficacy versus steroid-containing therapies, regardless of blood eosinophil counts. -
News Spiriva Respimat inhalation spray improves breathing for people with asthma regardless of BMI or allergic status
In all analyses, people experiencing uncontrolled asthma symptoms saw improvements in their breathing by adding Spiriva Respimat to an ICS or combination ICS/LABA. -
News FDA approves Genentech’s Actemra for giant cell arteritis
Sixth FDA approval for Actemra since its US launch in 2010, -
News Thermo Fisher Scientific to acquire Patheon
Patheon provides entry into the attractive, high-growth CDMO market. -
News Boehringer Ingelheim inaugurates biopharmaceutical manufacturing facility in China
It is the first and only biopharmaceutical manufacturing site established by a multinational active pharmaceutical company in China. -
News Hikma launches generic version on Edecrin in the US market
Product adds to the company's non-injectables portfolio in the US. -
News EMA validates application for BMS’s Sprycel in children with chronic myelogenous leukemia
Proposal extends application to the treatment of children and adolescents with chronic phase Philadelphia-chromosome positive chronic myelogenous leukemia and to the powder for oral suspension. -
News Biogen acquires Remedy Pharmaceuticals’ Cirara for large hemispheric stroke
Phase III-ready program complements Biogen’s ongoing development efforts in stroke. -
News Irvine Scientific launches new xeno-free medium for hematopoietic progenitor cell culture
New medium designed for hematopoietic cell culture for cell-based therapies. -
News Merck's CHOZN expression system selected for bi-specific antibody development by SystImmune
Accelerates development timelines with faster, easier selection and scale up of clones. -
News CPHI Worldwide to launch global pharma market ranking index
Research used to produce a global ranking of countries’ pharma reputation, market growth potential, innovation and competitiveness. -
News SGS expands stability capacity at Mississauga, Canada laboratory
Expansion part of global investment in testing capabilities. -
News Sartorius Stedim Biotech partners with Nova Biomedical
Unique tool will allow massive quantities of cell culture data to be collected during upstream processing QbD studies. -
News Saneca Pharma receives confirmation of multi-dosage cGMP approval for Russia
Certification covers the manufacture and packaging of hard and soft gel capsules, liquids for external use, semi-solids such as ointments and film coated tablets. -
News Friulchem S.p.A. Issued Patent for Rifaximin
Friulchem S.p.A., a chemical-pharmaceutical company active in human and veterinary fields, has received an US Patent (US Patent 14/11856) covering own solid form called “pseudo-crystalline” solid form, derived from Rifamycin O.
-
News Gamlen Tableting changes its name to Gamlen Instruments
New company name reflects diverse market opportunities. -
News Aptar Pharma’s electronic Lockout device approved by EMA
The e-Lockout device being the first and only fully integrated electronic nasal drug delivery device to be approved by a US or European regulatory authority. -
News Cambrex invests to increase pilot-scale API capacity at its High Point, NC facility
Increases site’s reactor capacity by 30%. -
News SGS Expands Stability Capacity at Mississauga Canada Laboratory
SGS has expanded storage capacity at its laboratory in Mississauga, Canada, for the effective stability testing of new drug substances and products for full regulatory compliance. -
News Catalent and Therachon to advance clinical development of innovative protein therapy for achondroplasia
Catalent Biologics will use its proprietary GPEx technology to produce different protein variants for Therachon, -
News Merck launches industry's first off-the-shelf cell culture media for perfusion processes
Allows customers to achieve a more optimal output than they would using conventional batch or fed-batch processes. -
News Research reveals manufacturers and contract packagers may miss serialization deadlines
Many companies currently unprepared because they lack the internal resources to devote to serialization. -
News Genentech to present new data on personalized medicines and cancer immunotherapies
New pivotal results for Perjeta and Alecensa demonstrating improvement over standards of care. -
News Avastin as effective as Eylea for treating central retinal vein occlusion
NIH-funded clinical trial shows use of either drug improved vision and had few side effects. -
News Hikma receives CRL regarding its ANDA for generic Advair Diskus
Company believes low likelihood of approval this year. -
News Merck's Keytruda scores another FDA approval
FDA approves Keytruda as first-line combination therapy with pemetrexed and carboplatin for patients with metastatic nonsquamous non-small cell lung cancer (NSCLC) irrespective of PD-L1 expression. -
News Takeda terminates plans for Vaxem Hib launch in Japan
Discontinuation due to the vaccine supplier’s (GSK) decision to discontinue global production. -
News New licensing program facilitates adoption of Tandem Mass Tag technology
Thermo Fisher Scientific helps research service providers and their customers optimize protein identification and quantitation. -
News Croda celebrates EXCiPACT Certification across all leading excipient sites
Status marks the company as the first multi-site excipient supplier to achieve global EXCiPACT accreditation across its production facilities. -
News Gyros Protein Technologies introduces next generation Gyrolab Protein A Kit for biotherapeutics
Kit increases efficiency of residual protein A ligand detection. -
News Roche's Tecentriq flops during Phase III trial
IMvigor211 failed to meet its primary endpoint of overall survival (OS) compared with chemotherapy.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





















































































.jpg)