- Home
- Packaging Materials and Equipment (Products)
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
Packaging Materials and Equipment (Products)
Packaging Materials and Equipment (Products) Companies (326)
Packaging Materials and Equipment (Products) News
-
News CPHI Online Trend Report – 2025 Pharma Packaging Prospects
The pharmaceutical packaging market has seen marked shifts in innovations, priorities, and focus over the last few years. With the rise of hit drugs like GLP-1 agonists and biologics requiring specialised packaging considerations, the strain of meeting...4 Apr 2025 -
News Inspiration, Innovation, Impact – Get Ready for Pharmapack 2025
This article goes into depth about waht you can expect to find on site at Pharmapack 2025, taking place in Hall 7.2 of the Porte de Versailles in Paris, France on the 22nd and 23rd January.5 Dec 2024 -
News CPHI Milan 2024 - From the Floor
Milan and CPHI welcome you to 2024 CPHI Milan! As we celebrate the 35th edition of our flagship CPHI show, editors Vivian Xie and Lucy Chard bring you the latest from the show floor, conference sessions, and innovative solutions from all exhibitors, at...7 Oct 2024 -
News Pharmaceutical Supply Chain People Moves
The latest appointments, promotions, and structural changes across the pharmaceutical supply chain.10 Sep 2024 -
News What can you expect from CPHI Milan 2024?
CPHI Milan 2024 marks the 35th anniversary of CPHI. The 35th Edition of the show, taking place 8–10 October, promises to be a celebration of everything pharma, covering every sector, and featuring thought leaders and trailblazers shaking up the i...25 Jul 2024 -
News CPHI Online Trend Report: what is pharmaceutical packaging really costing the industry?
The latest CPHI Online Trend Report dives into what it really costs to develop, manufacture, and deploy pharmaceutical packaging that balances sustainability concerns, patient safety, and drug efficacy.8 Jul 2024 -
News Gerresheimer predicts weight-loss drug deals to account for 4% of yearly growth
Dietmar Siemssen, CEO of German primary packaging manufacturer Gerresheimer, states that approximately 4% of the company’s revenue growth each year to come from deals with drugmakers of weight loss and diabetes products, particularly GLP-1 class ...2 Apr 2024 -
News Novel approach to creating sustainable packaging from rice husks
Researchers have created a new approach to the designing of eco-friendly nanofibres extracted from rice husks, addressing the critical need for sustainable packaging materials in food and biopharmaceutical products.27 Mar 2024 -
News Unpacking what we learnt: Key Pharmapack takeaways with L.E.K. Consulting
After another successful Pharmapack show, we sat down with Karin von Kienlin, Senior Partner at L.E.K. Consulting, to discuss the biggest takeaways and surprises from 2 days of intense discussion and collaboration.14 Feb 2024 -
News Pharmapack Awards 2024 Packaging Innovation Award Winner – Nissha Europe GmbH
The 2024 Pharmapack Awards celebrated the best in innovation and design for the pharmaceutical packaging and drug delivery industry on January 24, 2024.12 Feb 2024 -
News What did we learn from Pharmapack 2024? The green edition
Pharmapack Europe, held in Paris each year is a great opportunity to see the latest innovations and trends in pharmaceutical packaging and to hear from the companies and experts in the area on what aspects are most important to them in their products a...9 Feb 2024 -
News Pharmapack Awards 2024 Start-up Innovation Award Winner – Capa Valve Ltd
The 2024 Pharmapack Awards celebrated the best in innovation and design for the pharmaceutical packaging and drug delivery industry on January 24, 2024.2 Feb 2024 -
News Pharmapack Awards 2024 Patient-Centric Design Award Winner – Dr Ferrer BioPharma
The 2024 Pharmapack Awards celebrated the best in innovation and design for the pharmaceutical packaging and drug delivery industry on January 24, 2024.30 Jan 2024 -
News Women in Pharma: Minding the Gap at Pharmapack 2024
2024 marks the first year Pharmapack will host a Diversity track dedicated to bridging the gap within the pharmaceutical packaging and drug delivery sector. The track includes a panel discussion on 'Enabling Diversity in the Workplace,' focused...29 Jan 2024 -
News Pharmapack Awards 2024 - Celebrating Packaging and Drug Delivery Innovation
The 2024 Pharmapack Innovation Awards ceremony celebrated the best in pharmaceutical packaging and drug delivery innovation at all levels. The awards were held on January 24, 2024 at the Paris Expo Porte de Versailles.24 Jan 2024 -
News Pharmapack 2024 - From the Floor
Paris once again welcomes Europe’s leading trade show in pharmaceutical packaging and drug delivery innovation. Join our content team as Pharmapack 2024 opens its doors to leading experts and innovators in pharmaceutical packaging and drug delive...23 Jan 2024 -
News A Day in the Life of a packaging Project Engineer
To get to know the people working day to day behind our pharma companies, the ones who keep the wheels turning and ultimately bring better healthcare to the population, we would like to present a new interview series introducing pharma's talen...11 Jan 2024 -
News 2024 Pharma Industry Trends Outlook: Collaboration, Market Maturity, and Digital Futures
The annual CPHI Online 2024 Pharma Trends Outlook, in partnership with Arvato Systems, identifies 12 key industry trends shaping the life sciences industry in the coming year.9 Jan 2024 -
News On track at Pharmapack 2024 - The Track Sponsor interview: Gerresheimer
January 2024 brings both a new year and Europe’s leading packaging and drug delivery event. Gathering the world’s experts in pharmaceutical packaging together in Paris, France, Pharmapack 2024 offers exciting opportunities to learn and coll...2 Jan 2024 -
News CPHI North America 2023: from the experts – Sharp Services
Throughout CPHI North America (Philadelphia, PA; April 25–27, 2023), we caught up with some of the exhibiting organisations to ask what the show brings to the North American pharmaceutical sector, and what is driving industry innovation in this r...28 Jun 2023
Packaging Materials and Equipment (Products) Products (1000+)
-
Product Veterinary Syringe
Xinfuda specializes in the production of standard and customized veterinary syringes. 15ml 20ml 30ml 60ml syringes with wide nozzle are suitable for the oral of gels, creams and vitamin pastes to horses, sheep and pets. Our paste syringes consist of four components: barrel, cap, plunger and dosin...
-
Product SUSTAINABLE LABEL RANGE
- Customised sustainable labels on demand
- Recycled labels (Paper, PP, PET and PE range)
- Tamper ECO Paper labels
Find your successful solution with us!
-
Product Aluminium bottles - System Plus tm
• Capacity: between 50ml and 32L
A true market benchmark, the System Plus™ range is ideal for packaging, transporting and sampling sensitive products. Manufactured from aluminium 1050A and 1070A, this packaging provides high impact, tear and chemical resistance while also guaranteeing a 100%...
-
Product Cleanroom plastics injection moulding by GEMÜ
GEMÜ manufactures an extensive range of plastic injection-molded components, designed to meet the highest cleanroom standards for medical, pharmaceutical, and diagnostic applications. Our state-of-the-art facilities include partially and fully automated injection molding machines, operating in ISO Class 7 ...
-
Product Pouches
Oliver offers the most comprehensive set of pouch materials and custom design options for medical device and pharmaceutical packaging. Pouches are fully customizable--including size, design and material selection--for optimal protection and performance. A wide array of pouches are available including pouch...
-
Product PHARMACLEAN® Sterilisation and Packaging bags and reels
Pharmaclean® bags and reels are specialized products designed for contamination control in cleanroom environments, particularly in the pharmaceutical industry. They are made from DuPont™ Tyvek® material and PET-PP, which is known for its durability and resistance to moisture and punctures. These pouch...
-
Product Child-resistant dropper set with Tamper-Evidence
Newly developed and childproof, the ‘CRC-Pipette set’ from Heinlein guarantees certified product quality and highest safety standards. Our CRC pipette is mainly suitable for liquids that place increased safety demands on the user. Its ISO 8317 and US 16 CFR § 1700.20 certification guarantees that these...
-
Product High Speed Insulin Injection Pen Assembly Line
Customized High speed indexed assembly machine with 100% in line control
-
Product NOTIPLUS®
Our offer: an all-in-one label, NOTIPLUS®.
All-in-one packaging with integrated leaflet to optimize packaging design and reduce the carbon footprint of your productA multi-format leaflet, up to 25 pages, mono-material label, integrating 30% of recycling material (certified PCR)
&n...
-
Product DWK Life Sciences Pipette Forming
DWK Life Sciences is an established manufacturer of glass pipettes for the Diagnostics, Healthcare, Homeopathy, and Personal Care sectors worldwide. By leveraging DWK’s extensive manufacturing and development capabilities, we provide both standard and custom pipettes. Glass dropper pipettes are an excellen...
-
Product Corning® Valor® Vials
Valor® Glass Product Benefits:• Eliminates delamination
• Reduces glass particulate generation
• Resists damage and breakage
• Prevents* cracks
• Enables higher throughput through smoother filling line operations
• Decreases total cost of ownership
*In laboratory testing, Valor® G...
-
Product Paper Pouch for Pharma and Healthcare
Medicines and medical devices in paper bags? What works well for food could also serve as sustainable packaging in the pharmaceutical and healthcare industry. Faller Packaging has now developed paper pouches as a possible alternative to folding cartons. The Paper Pouch concept quickly moved from an ide...
-
Product CuraCase™ - Hypodermic needles (in Hard-Case Unit Pack)
Nipro is offering hypodermic needles in hard case packaging.
Benefits
• Optimized Processability • Smooth administrations • Secure device connection
Broad range • Outer diameter: 21 to 27G • Length: ⁵⁄₈" | 1" | 1 ¼" | 1 ½"
-
Product Vers-A-Tech™ - Cell & Gene Therapy Processing
Vers-A-Tech™ (Versatile Aseptic Technology) is a semi-Automatic or fully automatic modular versatile platform consisting of machines that come together to processes Bags & Nested Containers. BAUSCH Advanced Technology Group launches First of its Kind Platform Vers-A-Tech™ ...
-
Product Applicators
Bharat rubbers works manufactures Cream, Tablet & Gel Applicator in natural body & natural piston.
Cream, Tablet & Gel Applicator are massively used pharmaceutical applicators for treatments. They have efficient applicator properties for contraception, lubricants, spermicides, ant...
-
Product Analytical Lab Services
Analytical Services
The analytical services team has vast expertise and experience in extractables and leachables, particle analysis, container closure integrity, and performance and packaging/delivery systems among other methodologies. As a result of our understanding of materials and delivery ...
-
Product Vision Robot Unit (VRU)
We present a modular, robotic, proof-of-concept system for inspecting parenterals. By employing artificial intelligence principles and applying the technology to the inspection task, we engineered the Vision Robot Unit (VRU).
The VRU is a reliable, autonomous island machine, capable o...
-
Product Trays for autoinjectors made by Pulp-Injection
Customized trays, e.g. for self-injection devices
Pulp-Injection molded products have similar features and functions as plastic parts, e.g. high rigidness and
detailed design options.
The trays have dimensional accuracy and are stackable
to guarantee th...
-
Product Safe and compliant small diameter Polyfoil® tubes
Discover Neopac's world-renowned small diameter high-barrier laminate tubes from 10 mm / 1 ml. The tubes are available with several safety features, such as child-resistant closure and single-use options.
-
Product VhentPeel® Flat packaging made of grid coated paper
Our modular concept for grid coated paper includes:
• medical grade paper: 60 / 80 / 100 g/m²
• grid coating: 6 / 11 g/m²
Features:
• solvent free
• seals to rigid films and PE based sealing layers
• low – medium – high seal strength
• opt...
-
Product Pharma Packaging
Low in particles. Scratch-free. Unbreakable.
Unparalleled quality and safety - that's what you can count on with our tubs and nests. With our products, you can protect your sensitive pharmaceutical products reliably and cost-effectively. Our tubs and nests are manufactured under the strictest cleanroom ...
-
Product Foil for blister packaging
Symetal lacquered blister foils stand out for their compatibility to all different ink systems, while also offering excellent sealing performance against PS/PVC/PVDC substrates.
Accordingly, Symetal lacquered blister foil products serve as the perfect solution for pharmaceutical applications such as...
-
Product Epidural cannula
Sfm medical devices gmbh offers wide range of products which includes epidural cannula. It belongs to neurology / regional anesthesia / pain therapy category. It has developed a stable and functional epidural cannula for use in regional anesthesia. Special attention to the haptics of the neck and wing incr...
-
Product Container Closure Systems
Closures
In partnership with our trusted partners, we deliver comprehensive packaging solutions.
Our range of plastic closures for effervescent tablet tubes matches containers made from plastic, glass, or aluminum. Optionally featuring integrated desiccants, these closure systems ...
-
Product 4∞ Form
The 4∞ Form is a pharmaceutical blister pack that is made entirely of lacquered aluminium. The 4∞ Form is intended primarily for the pharmaceutical industry, where it can replace existing solutions used in packaging tablets, capsules and similar products, such as OPA/AL/PVC (cold form) or PVC/PVDC (thermof...
-
Product Primary packaging
PVC // AluPVC/PVDC // AuPVC/PE/PVDC // AluAlu // Alucontrolled substances packagingpackaging of potent OSDsbottles (glass, plastic, aluminium) with different caps (push-on, screw, snap safe, desiccant capsules)unit dose blister
-
Product BFS One-Stop Partner
The Rommelag Group sees itself as a One-Stop Partner for Blow-Fill-Seal Technology and a specialist for flexible Containment Solutions. With our complete solutions for Fill&Finish, we are a strong partner for the pharmaceutical, food, cosmetics and chemical industries.
-
Product Merieux NutriSciences - Medical Device services
Thanks to a valuable pool of experts, Merieux NutriSciences supports you in the development of the testing plan to determine which studies are necessary to ensure that the device is safe and effective, meeting the essential requirements for affixing the CE mark on the product. Download our brochure: ht...
-
Product Serialisation
Serialisation and Track & Trace Addressing evolving global serialisation legislation, Almac's expert multi-disciplinary serialisation team lead the way in supporting clients in developing their serialisation strategies to GS1 standards. Our Experience Almac have over...
-
Product Taste Masked Coated Granules of Clarithromycin & Azithromycin
White to off white tasteless free flowing granules Recommended for Dry Syrup / Suspension
-
Product Development & manufacture - drug delivery devices
Bespak by Recipharm delivers market leading design, development, and manufacturing services for drug delivery devices to the global pharmaceutical market. Our portfolio includes inhalers, nasal technologies, and auto-injectors as well as development and manufacturing services.
-
Product DIAGNOSTICS | Customer-specific plastic consumables
S-TubePCRTube with screw-cap, mass production with integrated assembly process and leak-testing system. Material: >PP<, >SB<
-
Product Rubber Punched Gaskets And Punched Plastic Gaskets
RUBBER PUNCHED GASKETS: With 70 years of experience in manufacturing punched parts, TekniPlex Consumer Products is a global market leader in aerosol industry applications. This experience makes us an ideal partner for manufacturers and co-packers who need a precision part, with tight tolerance for their de...
-
Product Blister serialization
Although primary pack serialization is not yet a mandatory requirement, it is important to consider the benefits of adopting this technology in view of current healthcare industry production scenarios. Firstly, today the technological evolution of the digital printing industry and the development of new ha...
-
Product PN skin booster (Lilied)
DNA Healer as medical device / Polydeoxyribonucleotide + Lidocaine / 2ml
-
Product Pollen & Pollution Catcher Nasal Spray
With Moisturizing & Soothing Mallow Prevents main allergy symptoms while keeping nasal mucosa soothingly moistened
-
Product Combo Excelsior Hybrid
With a minimized number of parts, its UV resistant material, the Combo Excelsior Hybrid® is suitable for all harsh industrial environments and can stay stored outside on a long time range. Improved bottom discharge zone speeds up the liner and valve set up and removal. Dustcover on discharge zone ensures m...
-
Product PHARMA PE BAGS (ATEX compatible)
PE unit bags for food ans pharma packaging. Ph/USP/FDA approved. Dimensions ranges from 100 to 3000mm, with or without gussets. Different sealing and formats availables.
Can be manufactured with L2 special resistivity specifications, to prevent explosions in ATEX zones.

-
Product Packaging & Labeling Solutions
At WuXi STA, we understand the need for flexibility at the early stages of clinical testing and how aspects such as effective dose, stability and compliance requirements can change your needs abruptly. Our packaging team will work with you to collect the relevant data and test various packaging options as ...
-
Product 2K single-dose strip
This innovative product stands out for its unique feature - a cap that protects the spout, significantly minimising the risk of contamination. This development represents a leap towards safer, more reliable dispensing solutions for a variety of applications. Perfect for ophthalmic and pharmaceutical li...
-
Product Pharmaceutical Rigid PVC
PVC is available in a wide range of colors and thickness. PVC rigid sheets can be used in packing capsule tablet pill, with high chemical stability, fine anti-fire, supertransparent,high hardness.
-
Product Siliconized Vial
Chongqing Zhengchuan Pharmaceutical Packaging Co. Ltd provides wide range of pharmaceutical glass tubes which includes low borosilicate glass tube. Contact us for more information.
-
Product SELF ADHESIVE HANGING DEVICE
The Self Adhesive Hanging Device for infusion bottles is one of the latests Pilot Italia patents.
Our R&D team has developed and patented three labels with an innovative handle and various functionalities, aimed at simplifying and supporting operating room staff while meeting custome...
-
Product Flexible Films
Our flexible films are provided with their specific properties according to their coextrusion and lamination processes. Typically, the material structures are designated for highly automated processes. Most films can both be used as base material and lidding material.
-
Product PLASTIC BOTTLES WITH TAMPER-EVIDENT BAND
Plastic bottles (plastic containers) with tamper-evident band for pharmaceutical use are used for packing (storing) liquid pharmaceutical products (magistral medicine, galenical medicine, ready-made medicines, and pharmaceutical substance). They can be in direct contact with the product. These containers a...
-
Product TECOBOX: the ultimate tamper evident box
TECOBOX enhances pharmaceutical packaging security with design that visibly indicate tampering, thereby ensuring product integrity and compliance with industry standards.
The TECOBOX seal is part of the box and needs to be ripped-off to open the box. That's irreversible and also evident. qq...
-
Product Ophthalmic and lenscare: Tamper-proof dropper bottles
• Bottles with screw-on neck finish • Compatible with Röchling droppers & closures (3 part system) • Dropper Ø from 0,25 mm–1,2 mm (up to 2mm possible) • Tamper-proof closure, various colors available &...
-
Product Multipage Labels
The original multipage self-adhesive label, zig-zag or booklet folding, creates additional space to convey methods and instructions for use, warnings, legislative references, product range and manufacturer’s information. Adaptable to any packaging type, flat or cylindrical, can be supplied with adiffe...
-
Product CAPS FOR ALUMINUM TUBES
Closures for aluminum tubes are especially used in the field of pharmaceuticals for creams and ointments. A wide range of sizes and colors is available in the standard area. Optional also with piercing mandrel available.
-
Product Deep drawn caisters for SMI cartridges (Soft-Mist Inhaler)
Pressteck is a specialist for the production of deep-drawn SMI cartridges made of aluminium or other metals. With our long-standing experience and our reputation for highest quality, we are a strategic partner to some of the leading pharmaceutical companies.Our cartridges are used as primary packaging ...
-
Product Ready To Use Platform
Our Ready To Use is available for - Prefillable syringes: 1ml standard, 1ml long, 2.25ml, 3ml
• Vials 2R, 6R, 8R, 10R • Cartridges: 1.5ml, 1.8ml, 3ml • Cop Syringe • COP Vials. - We use borosilicate type 1, 51 expansion glass
ISO 13485 | ISO14001 | ISO15378 | ISO45001
...
-
Product Safe and cost-efficient packaging of pharmaceutical products
After introducing a new medication to the market, it is always a challenge for pharmaceutical manufacturers to calculate production capacities without incurring economic losses. With support from machine manufacturer Schubert-Pharma and packaging specialist Faller Packaging, manufacturers now have two stro...
-
Product Z-810 Automatic robotic platform
The Z-810 robotic platform is a state-of-the-art solution, specifically engineered for automated aseptic handling and fill-finish of Ready-To-Use (RTU) syringes, cartridges, and vials.
This advanced modular technology is designed to mitigate any potential microbiological contamination, while sim...
-
Product Shrink Sleeve
Revolutionize Your Packaging with Etiktech Shrink Sleeve Labels
Etiktech, an expert in innovative packaging solutions, presents its Shrink Sleeve Labels! Protect, secure, and personalize your products with our high-end shrink sleeves.
Our Solutions:
• Plain sleeves for a s...
-
Product ABC Transfer® Alpha Port
Our range of rapid transfer ports, ABC Transfer’s Alpha Ports, ensure safe sterile and aseptic transfers for isolator systems in pharmaceutical filling lines. Our Alpha ports, rapid transfer port are compatible with other brands and fully comply with the Annex 1 of the European GMP.
...
-
Product Applicator
What makes an Applicator interesting? • controlled, precise application of the product • accurate control of the product flow by choosing the spring tension, the opening hole and the cover material • suitable for a variety of applications, e.g. acne therapy, insect repellent, sunscreen, pimple treatment ...
-
Product Empty Hard Gelatin Capsules
ETO FreeTiO₂ Free
Halal Certified
Kosher Certified
ISO9001
ISO22000
EU Registration
-
Product Ophthalmic CDMO Services
Salvat offers the best services and solutions, drawing on a long history of expertise in developing and manufacturing successful medications.
Tailored solutions to customer needs through our Ophthalmic platform: • • Customized solutions using Salvat's technology: suspensions, solutions, micellar,...
-
Product 1ml clear glass ampoule
body dia :10.25mmtotal height :60mm
body height: 25.5mm
color: clear and amber
-
Product Advanced PolyPropylene and PolyEthylene Multilayer Infusion Bag films
PolyCine Standard
Multilayer Film for infusion bag production
Packaging for Antibiotics, Anti-Viral Drugs, Haemodialysis Solutions, CAPD Solutions, Standard Infusion Solutions, Plasma-Volume Expander, Anti-Infective Drugs, Analgesics etc.
Customized widths, roll lenghts etc.
qq...
-
Product Ophthalmic Dropper Bottles
Main Features of Our Ophthalmic Packaging: • Customizable drop size, packaging sizes and colors • Drug Safety: Variety of Colored Screw Caps Can Help Distinguish Multiple Formulations, • Air-tight and light-tight white packaging - Leak tightness test has been carried out • &...
-
Product AL/PE Strip Foil
Easy tearing; Antioxidation; High barrier; Heat sealing; Good sealing; Printable.
-
Product CAPD (CONTINUOUS AMBULATORY PERITONEAL DIALYSIS)
- Polyolefin based 3 layers and co-extruded film - Excellent heat seal property & good mechanical strength - High impact resistance at low temperature - &n...
-
Product Extractables & Leachables Testing
Nelson Labs Europe offers a comprehensive approach toward Extractables & Leachables testing for the pharmaceutical industry. Our approach combines technical and analytical expertise, polymer knowledge and understanding of regulatory requirements, all combined in a tailored approach for our customers.
-
Product Pharma PVC/PVdC blister film
Thermoformable, US-FDA compliant, Pharmaceutical - Grade two layer PVC/PVdC film. Yash Packaging offers a wide range of PVdC-based film structures to meet both performance and cost requirements with moderate to high barrier blister films.
-
Product Analytical Services & EU Batch Certification
Method Development and validation
Stability Studies
European testing & batch certification

-
Product Pharmaceutical Label Products
CCL Healthcare offers an extensive range of top-quality secondary packaging and labeling solutions. Developed by leading designers and engineers, our products lead the industry in quality and innovation. Tested thoroughly, available in various configurations, and customizable, we incorporate innovative fea...
-
Product SENSOkidCAP®
SENSOPLAST® combines the advantages of tamper-evident closure with a child safety solution. The cap can only be removed from the bottle by simultaneously pressing and turning the cap. At the same time, tearing off the tamper-evident ring reveals the initial opening.
This special design has prove...
-
Product Serialisation for pharmaceutical company packaging
Sometimes a tour of De Budelse leads to progressive insight and a request for complex printed matter. As a specialist in packaging, we love challenges, and
we like researching solutions for new customers. Especially if that customer belongs to one of the largest pharmaceutical companies in the world. ...
-
Product BlisBa - PVDC
PVDC coated films for blister applications
• for low to middle barrier demands • Duplex (DX) and Triplex (TX) structures
-
Product BlisterJet 807
Digital blister printing system for late stage customization of blister production. Available with up to 2 spot colors. The BlisterJet is a fully digital piezo inkjet system. It prints on blank blisters. The solvent-free, UV-curable inks print sharp graphics and text in one to two colors. The system i...
-
Product Services - Lab analysis
We offer the quality standards that we apply to our own environment, machines, solutions and processes as services to our customers as well.
We make continuous investments in our specialist expertise. Quality assurance is the focus of our work. To ensure that we can meet your requirements r...
-
Product CLOSING TE
Bormioli Pharma offers a wide range of glass bottles for ophthalmic products which includes closing te. It belongs to ophthalmic dispenser category. Contact us for more information.
-
Product Aluminum Tubes
TUBE is equipped with the latest European technology to provide the finest quality, and the largest regional production capacity of aluminum tubes covering the diameters of 13.5 mm, 16 mm, 19 mm, 22 mm, 25 mm, 28 mm, 30 mm, 32 mm, 35mm, 38mm and 40 mm.

-
Product Labels
FEATURES - All printing technologies (Offset, Flexo, Screen and Gravure)
- Combination printing up to 14 colors
- Leading variety of digital printing options, incl. variable data printing for serialized fully...
-
Product EcoSecur type 2 glass
Stoelzle Pharma reached another milestone by developing a new, safe and resource-efficient process for the inner surface treatment of type 2 glass vials. This innovative technique enables reliable and precise dosing tailored to each bottle size, from the smallest 6 ml vials to much bigger. With EcoSecur in...-comp241444.jpg)
-
Product Dropper bottle set 5 ml, 10 ml, 15 ml with cap Ø14 mm
Purpose: direct packaging for liquid products
Material:
bottle – LDPE
dropper – LDPE
cap – HDPE
• various colours on request

-
Product FEXUCLUE®
Fexuclue (Fexuprazan) is the best-in-class novel anti acid secretion agent with rapid onset time and potent acid suppressive effect, addressing the growing unmet needs of PPIs
Highlights of Fexuclue are: * Launched in Korea (2022) and Philippines (2023)
* Approved in Chile and Ecuador qq...
-
Product Visual Inspection Table CLEANVIEW©
The luminous intensity of the new CleanView manual inspection table is ensured by independently adjustable LED strips which allow a more homogeneous luminous intensity than neon lights, more economical and ecological with a lifespan of + 20 000 hours visual inspection.
The design of th...
-
Product DWK Life Sciences Primary Packaging Solutions
DWK Life Sciences is a leading global manufacturer and supplier of glass and plastic primary packaging. Our Primary Packaging container closure systems are manufactured from the highest quality pharmaceutical-grade tubing designed specifically for pharmaceutical, biotech, diagnostic/IVD, and personal ...
-
Product Production in class 7 clean room and Brickpacking
We offer an extensive range of PET bottles and jars that we can produce under Cleanroom conditions. Our Cleanroom production corresponds to Class 7 according to ISO 14644-1
-
Product VITALDOSE® PHARMACEUTICAL GRADE ETHYLENE VINYL ACETATE (EVA)
VitalDose® EVA copolymer is a technology platform for reliable sustained release performance in indications requiring long-acting drug delivery. Common applications of this ethylene vinyl acetate pharmaceutical grade copolymer include subcutaneous and ophthalmic implants and intravaginal inserts. Ther...
-
Product Nanopass™ 34G Pen Needle
Nanopass™ 34G Needle for Pen Injector features a 22% thinner double-tapered needle aiming at a better flow than comparable 32G designs.
-
Product Brominated butyl rubber seals
Hebei First Rubber Medical Technology Co offers a wide range of products which includes brominated butyl rubber seals. Application: suitable for the seals of antibiotics and other powder crude drugs. Packing: inner double sterile plastic bag, outer corrugated carton. Contact us for more information.
-
Product Mikron MAIA - low volume assembly and test system to produce single or multiple variants
The Mikron MAIA is a low volume assembly and test system to produce single or multiple variants of medical devices such as auto-injectors, pen injectors or syringes. All key assembly processes are automated to ensure quality and data collection. The station parameters can be re-used for later productio...
-
Product MK™ Fine Mist Sprayer
Deliver precision dosing and superior spray performance for your Rx and OTC medications. Meet MK™—a fine mist sprayer designed to deliver precision dosing and superior spray performance. With its excellent priming, atomization, and clean output, it delivers an effortless spray experience for consumers. At...
-
Product CordenPharma Fully-Integrated Supply
CordenPharma’s vertically-integrated supply chain model provides development & manufacturing expertise spanning the complete GMP supply chain. Beginning with regulated Key Raw Materials and Non-GMP Intermediates, our sister organization WeylChem supports back integration to sec...
-
Product Container Closure Integrity Tester for laboratory use
With our wide range of lab testing equipment we provide solutions for the early stages in the development processes as well as for quality control in commercial manufacturing. Being able to transfer the test method from R&D to production reduces risks and saves time.
The NEO series combines high...
-
Product Syntegon´s Solutions for the pharmaceutical industry
· Process technology
· Solid dosage forms
· Liquid dosage forms
· Inspection technology
· Secondary packaging...
-
Product Alu-Alu Foil (Cold Form Foil) ALUBLIS
Alu Alu Foil is most widely used in the pharmaceutical industries, and with Svam Toyal Packaging Industries Pvt. Ltd you get the best in class quality at most competitive rates.
Alu Alu Foil is available in 45 & 50 Mic. Aluminum foil offering better mileage and cost saving to the customers...
-
Product Aidaptus
Aidaptus has been awarded the Red Dot award for the category Product Design 2023
Benefits
- Aidaptus® is a 2-step single use auto-injector platform, with a versatile design and intuitive drug delivery.
- Aidaptus® accommodates both 1mL and...
-
Product Cold-forming Aluminum
Cold form blister packaging is especially useful for drugs that are sensitive to temperature, light, or moisture, as it helps to maintain the stability of the drug by providing an airtight seal. The blister packaging is also easy to open, making it convenient for patients to access their medicati...
-
Product Sustainable labeling solutions
Even though all pharmaceutical packages can’t currently always be recycled, there is always a way to increase the sustainability of the package and cause less burden to the environment by choosing labels manufactured from renewable or recycled raw materials, or solutions that are resource optimized and...
-
Product Folding Cartons
High functioning folding cartons offer an innovative, sustainable and cost-effective packaging format.
- Ability to apply Coding and Serialization - printed invisible, black light or IR inks or combinations
- Embossing including Braille to one or all panels.
- Tamper-evident and child-pr...
-
Product Customized silicone syringe plungers by RAUMEDIC
Syringe plungers and stoppers made of our self-developed, slide-optimized silicone that comes in medical-grade quality have low break-loose force. This enables injections to be controlled and dosed much easier.
• Clean manufacturing conditions • Freedom of design and shape when using silicone • Small...
-
Product GREENIS® DISPENSER
This amazing dispenser is made of high content PCR PP resin and is RecyReady©.
Its attractive and intuitive way of applying cares presents a real advantage compared to many packages available on the market.
Designed as light as possible, using post-consumer resins or materials that are recyclable...
-
Product Shrink Tack Label - Light Protection
Our Shrink Tack Label now comes with a light protection function! Depending on your requirements, you can choose films from our substrate line-ups.
For example, the clear type allows the content to be visible, but your light sensitive drug is still protected from UV light, up to 380 nm of wav...
-
Product End-to-end contract manufacturing makes it simple
Only a true end-to-end partner can really simplify the entire manufacturing process. • One partner that works with you from product development and prototyping to full production.
• One partner with the metal and plastic expertise to make your complete device from the handle to the sharp “bus...
-
Product Folding Box
The best packaging is the one that combines accurate planning, effective design, the ability to serve the specific communication needs of each product and an impeccable technical realization for printing, folding and set-up. The production cycle uses 5 sheet-fed presses, 8 die-cutters and 10 folding/gluing...
-
Product The Blue Tube Evo
The Blue Tube Evo was the world’s first tube to be made of 100% recycled aluminium. And it closes the loop by incorporating aluminium packaging that has been used and thrown away by end-consumers. The Blue Tube Evo is the result of an innovative and future-focused eco-design approach. It offers a wealth of...
-
Product Full solutions provider
Nolato is a global full solutions provider and contract manufacturer specializing in product and process development through final device assembly and fulfillment. Our offering covers the entire value chain, from development to high-volume production and beyond.
Product development and design for ...
-
Product Sycare
Sycare is a pre-fillable syringe in Cop. It is the only one on the market to also have a threaded cap made of COP.
The threaded cap is unique and patented. It provides a remarkable sealing power, combined with an exclusive design. Sycare has been designed to be recognisable and to distinguish t...
-
Product Flip-OFF-SEALS, SAFE DROPPERS, ADAPTERS, MEASURING CUPS, THERMOFORMING BLISTER COMPONENTS, SPATULAS, MASTERBATCH, OPHTHALMIC CONTAINERS, CANISTERS, ORAL SYRINGES, TYVEK PRINTING & INDUCTION SEALING WAD,
SAFE SEALS: DMF Grade Sizes : 13mm, 20mm, 28mm and 32mm
Colours : Can Match any colour per requirement
Finishes : Glossy and Matt. Custom Instruction Printing. Custom Logo Embossing.
Single...
-
Product Atec Transfer Port - ATP
The Atec Transfer Port acts as a closed transfer lock to enable sterile transfer of components into and out of isolators and RABS. A unique locking system was developed to guarantee both operator safety and product integrity. The ATP cannot be opened unless the Beta port is correctly docked. This also mean...
-
Product INVISTA medical polypropylene resins
MELITEK offer and distribute PP resins in Europe from INVISTA (former Flint Hills) USA, who has a wide and unique range of medical PP materials. INVISTA has for many years been leading supplier of medical PP in US market. We have proudly serviced the product range to European customers for over 30 year...
-
Product Plastic injection molds
Mold design and manufacturing for Medical and Diagnostic in Vitro plastic devices.
-
Product Biosafe® Simplifies Your Transfer of Closure Components
Secure Your Aseptic Transfer
With the robust design and proven performance of the Biosafe® RTP system (Rapid Transfer Port) you can secure and simplify the transfer of your components, monitoring tools and liquids into your isolated line. Biosafe® bags exits in RTU (Ready to Use) and R...
-
Product ProMoCurve - Assembly platform for medical equipment
The ProMoCurve from Strama-MPS is an assembly platform for a wide variety of consumables in the life science industry, such as
inhalers, insulin pens, lancing devices or laboratory disposables. It is based on a modular platform that can be expanded almost at will.
This means that we a...
-
Product Vial Adapter Ø13 and 20 mm
A full range of Vial Adapters devices for drug transfer and reconstitution, in blister, with or without filter 5 or 15 micons.
Contact us for more information.
-
Product Injectable vials in molded glass - Clareo
Clareo, the highest quality Type II premium injection vials Clareo, the highest quality Type II premium injection vials Clareo vials are made of Type II glass and offer a combination of homogeneous wall thickness with superior cosmetic qualities suitable for high value products. Available fro...
-
Product Device Design & Development
From initial concept design to industrialization, Jabil Healthcare Engineering & Technology (JHET) + our wholly owned subsidiary Radius Innovation & Development help the most trusted healthcare brands to solve their increasingly complex product design and development chall...
-
Product Smart hearing health
TympaHealth, one of our start-up clients, launched the world’s first all-in-one hearing health assessment system, a portable smartphone-enabled otoscope with accompanying smartphone app. Since then, the novel device has been awarded Gold in the internationally recognised UX Design Awards for its innov...
-
Product Small embeddable RFID micro tag for Syringe, Vial, Cartridge Serialization
Product Brief
The RFID micro tag can be fit on or embedded into any metal/non-metal objects, such as rigid needle shield, and rubber plunger.
This product can be used globally with high performance and reliability.
Why is RFID necessary for Pre-f...
-
Product Compression Springs
Advanex produce a wide range of compression springs and from 0.1mm to 3mm wire diameter in a wide variety of materials from carbon spring steels to alloy wires. With full inhouse design capability, our Technical Department can help you find the optimum design for your device, ensuring your product is no bi...
-
Product ESD-Staticare®
Antistatic Cleanroom Packaging to ensure perfect protection of products
Characteristics:
-ESD - permanent antistatic
-flexible LDPE-film
-Cleanroom Production: GMP Class B
Staticare® - migratory - antistatic properties -...
-
Product Biocompatibility - E and L Studies for Medical Device or Combination Products
To ensure patient safety, precise knowledge about potential contamination associated with medical device materials is critical. For this reason, a robust chemical characterization is the first and mandatory step of any biocompatibility study which is performed according to the most recent version of the IS...
-
Product Pharmalene LDPE
Versalis, as a global producer of PE resins, provides knowledge & expertise to the pharmaceutical and medical market since decades. This wealth of experience allows Versalis to provide complete and complex solutions to a highly demanding and evolving market. Versalis recognises the key requirements ...
-
Product Datwyler's components for Vial applications
Datwyler is the preferred solution partner to global pharmaceutical companies. We offer one of the most extensive product portfolios for vial applications in the pharmaceutical and biotech markets worldwid. Our range of products and services includes the most innovative elastomer formulations, coatings, al...
-
Product Pharmaceutical, cosmetic and nutraceutical products
PRODUCTS
We adapt to our customer’s specifications. Our factories have been designed thinking in the pharmaceuticalmarket. All the production processes are performed inside clean rooms,with ventilation systems equipped with H13-type absolute filters, toensure the complete absence of external contamina...
-
Product Injection / Thermoforming Contract manufacturing
Injection / Thermoforming Contract manufacturing
-
Product BLISTER PACK TECHNOLOGY
An innovative patented strengthen blister pack to store fragile forms such as ODTs.
A three layers blister (Al/PVC – PVC – PVC/Al/PA)
The intermediate PVC layer serves as push trough device + additional reinforced pocket
European, US and Canada paten...
-
Product PEKUWhite - Cleanroom packaging - PE bags / films / tubes
PEKU Folien - Cleanroom and Pharmaceutical. Your Packaging. Our Passion. Film extrusion, Converting, Co-Packing according ISO 9001 and DIN ISO EN 14644 grade ISO 7 / EU GMP -B in operation, ISO 5/EU GMP- 5 at rest;
PEKUWhite: Tublar film, center folded film, flat film, bottom seam bag, ...
-
Product DROPPER BOTTLES
For Special Cap and Pipette
For D30 Cap and Pipette
http://www.neutroplast.com/en/products/dropper-bottles/
-
Product Pharmaceutical leaflets
Intrograf has a separate production area dedicated to leaflet production with sustained conditions and continuous control of such parameters as humidity and temperature. This allows us to optimize production and to print and fold low grammage paper leaflets of various sizes and folding styles.
-
Product ClearVial - COC or COP vials
MEDICOPACK A/S provides a wide range of vials which includes clearvial - manaufactured from COC (COP only available upon special demand). It is transparent, lightweight and with good impact strength, clearvial is a breakthrough in primary packaging solutions for the pharmaceutical and biotech industries. M...
-
Product Secondary Pharmaceutical Packaging
Secondary Pharmaceutical Packaging
Cartoning Machines
Clocked horizontal cartoning machines for folding boxes, also with 5th flap Solo or inline systems
Leaflet folding for package inserts, max.: 210 x 600 mm
Coding, code reader, data matrix code (with / without serial number...
-
Product GasSpect O2
The Gasporox sensor series GasSpect offers non-destructive Headspace Gas Analysis to be integrated into an inspection machine. The sensors with its highly sensitive laser impresses with performance and precision. The GasSpect series comes in different variations depending on which gas to be inspect...
-
Product INTRAOCULAR LENS INJECTORS
Ophtamologic injector with a body, a pusher, a metal spring and a sleeve (plunger).
-
Product ACLAR ACCEL™
WHEN “GOOD ENOUGH” IS NOT ENOUGH, ACLAR ACCEL IS THE PERFECT GO-TO SOLUTION.
Joining our flagship line of Aclar films, Honeywell Aclar Accel is a high moisture barrier thermoformable film product that delivers at the price and speed business demands while providing the quality and servic...
-
Product Applicators
Elm-plastic GmbH offers a wide range of products which includes applicators. Features: it is used for oral application of vet.-med. Pastes and gels. The ergonomic shape of the elm-plastic applicators guarantees comfortable and secure handling. The special geometry of the piston ensures an unproblematic fil...
-
Product Package inserts
As a recognised specialist in package inserts, we are the perfect partner for virtually any package insert project. From simple, monochrome inserts to outserts and double or triple leaflets with single-colour or multicoloured print, we produce and fold your chosen products to create the sophisticated combi...
-
Product BAIA
BAIA line:- Metering pumps with doses from 0,25 to 0.5 ml
- Bottles: from 15 to 50 ml
- Pouches: from 15 to 50 ml.
Contact us for more information.
-
Product Closure 1.4k (DIN18)
Closure 1.4k is one-piece screw closure, which guarantees reliable product protection against counterfeiting and accidental tampering.One-piece covers with a protective ring are easy to use and provide tightness, as well as strong fixation.
It can be equipped with various droppers.
Pharm...
-
Product RayDyLyo®
RayDyLyo® is a new standard for vial capping. Alternative solution to aluminum crimped cap, RayDyLyo® is a patented range of plastic press-fit closures, solving long-standing issues in aseptic production from the capping process and from cosmetic defects. RayDyLyo® is a breakthrough innovation, whi...
-
Product covers // NORMA
"NORMA" is literally the benchmark for lidding foils.
- A wide variety of aluminum layers, in hard or soft temper, dull or bright
- Different primers for excellent in-line and off-line printability
- A wide selection of sealants to bond with PVC, PVDC, PP, PE, PET, COC, PCTFE
- Preprinted f...
-
Product Assembly and test systems for medical disposables / plastic assemblies
Dispensing pumps, stopcocks, tubing sets and other medical disposables are mass-produced and marketed in huge volumes. We have developed the fast-cycle TEAMED RTS assembly system especially for this purpose. Its mechanical cam drive guarantees stable processes and high volumes for decades to come - 2...
-
Product Complete lines for sachet and stick-packs in cartons
Universal Pack Synthesis complete automatic packaging Lines are individually custom-built, whether for primary or secondary packaging, guaranteeing the highest efficiency together with the most compact machine layout, perfectly in line with Universal Pack traditional concepts.
-
Product Medical devices for gynecological application
INGE produces medical devices for vaginal liquid products, creams and suppositories. 50 ml to 180 ml bottles for vaginal shower made of a soft, easily compressible material, also Green. Bottles are available in various shapes - cylindrical or elliptical - customizable with front and back engraving and one-...
-
Product Syringes packaging solutions
Products advantages : • Customized solutions to fit yours needs
• Secure closure, No touch packaging ...
• Sterilizable : gamma or bêta rays, steam
Our standard range includes no touch blister in PETG, syringes boxes, folding blister, multisyringes shell PC, customized velbox...
-
Product Pentapharm® BlisterPro® XCEL Services
Imagine design and prototyping Services that enable accelerated market introduction with increased accuracy in stability testing using the most cost-effective blister film to meet your requirements. Contact us for more information.
-
Product Healthcare Polymer Solutions
Our wide-ranging product portfolio includes brands by well-known polymer manufacturers. We are therefore able to provide a wide variety of different plastics that have been specially developed for the healthcare sector.
-
Product Contract Assembly and Packing
We can support you in:
- Automated and Manual Product Assembly & Kitting
- Blister Filling and Sealing
- Pouch Filling and Sealing
- Form Fill Seal Packaging
- Labelling
- Secondary & Tertiary Packaging
- (Pha...
-
Product plastic primary packaging
closures and containers for pharmacy, CRC and TE caps, push on TE caps, containers with screw on and push on thread
-
Product CombiSys
// Flexibility meets zero reject principle. ///
CombiSys is an efficient and highly productive system solution that enables you to process various packaging materials on a single platform. The transport system uses grippers that give you a wide working range without changing size parts. Modul...
-
Product GSS P Steam Sterilizer for pharmaceutical production
GSS P is a steam sterilizer from Getinge for component sterilization in pharmaceutical production. GSS P ensure a reproducible process, product quality and a safe environment. This makes it easier to achieve high performance, productivity and a streamlined process.
Getinge develops, manufactures a...
-
Product Plastic bottles and closures
Drug Plastics has been designing and manufacturing HDPE, LDPE, PP and PET bottles and closures for over 50 years. Our products offer the highest level of material control and process integrity; and we manufacture all of our pill bottles, plastic liquid bottles, and complementary closures with relentle...
-
Product Tyvek®
Tyvek® coated or uncoated, is a breathable material made with pure polyethylene fibers, which guarantees an optimal protection from microbial penetration, against tearing and perforation.
Tyvek® is characterized by an excellent peel ability during opening a medical device, if welded with peela...
-
Product CIRCULAR CARBON MANAGEMENT
We are constantly exploring new possibilities to enable future viable solutions for the healthcare sector. We strive to minimise our carbon footprint by improving recycling processes and undertaking research into sustainable materials and resource saving production cycles.
-
Product Cartoning Machine MA300 - 400
Continuous motion horizontal cartoners for packing any kind of pharmaceutical products at high speed.The perfect balance between reliability, safety and performance.
Main features:
- Versatility and efficiency in a small footprint- Balcony design
- Oil-bath main drive units
- ...
-
Product Primary packaging for biologics
Primary packaging for sensitive, biotechnologically manufactured active agents. Highest quality standards with regard to active agent-packaging interaction, increased user-friendliness and application safety for patients and user.

-
Product Divider in solid PP
Interlocking divider obtained from solid PP sheet, die-cut and assembled.
-
Product Thermoform Packaging Machine: RX 4.0
Innovation at its best !
The RX 4.0 offers maximum process reliability. Using closed control circuits, the sensor system constantly captures all the relevant process parameters, such as those for forming, evacuation and sealing. All the process stages are coordinated to the optimum level...
-
Product Supply Chain Management: P24
A complete system for the production in 24 hours and “Just in Time” delivery of secondary packaging for the pharmaceutical industry. Equipped with digital printing technology and integrated with 100% automatic visual inspection systems, the P24 enables on-demand production of folding cartons, leaflets and ...
-
Product Packaging in Blister
Primary packaging: Forming station for both PVCs and aluminium and all kind of feeder for tablets and capsules. Secondary packaging: Labelling, carton and casepacker.
-
Product ALUMINIUM PILFER PROOF CAPS
suitable for pilfer proof bottle mouth, and available in diameters from 17 mm up to 38 mm
different colours, different logos different liners or insert (like dessicant)

-
Product Polyvinyl chloride solid medicinal hard film
production process
It is made by mixing, extruding plasticizing, calendering and slitting with polyvinyl chloride resin as raw material and various functional additives. Medicinal blister packaging materials are mainly used for solid oral preparations(tablets, capsules, etc.)
Features qq...
-
Product Packaging and labeling
Alcami provides custom packaging, labeling, and kitting for clinical trial materials destined for trials anywhere in the world. We also provide specialized expert support for randomization and blinding studies, including placebo, comparator, and crossover studies, as well as reconciliation drug accountabil...
-
Product SERIALIZATION
We offer total expertise for the serialization of your individual products. For that we use the most modern technologies available for printing, packaging and labelling solutions. Our intelligent software systems ensure a smooth serialization throughout the manufacturing process, which guarantees...
-
Product Health Economics & Value
Even if your objective seems clear–demonstrate the value of your product – it is rarely a simple task. That is because there is no single story or piece of evidence when you speak to audiences around the world: regulators, health technology assessment (HTA) bodies, payers, providers and patients. IQVIA ...
-
Product Omag Packaging Machine mod. CP
Intermmitent motion packaging machine with sealing plates technology to guarantee perfect sealing and high quality of the sachets, without any wrinkles in order to maintain the integrity of the product. Possibility to pack different products in classic 4 side sealed sachets or shaped sachets. The idea...
-
Product Lab Laser Drilling Machine
This machine is designed to penetrate the outer coating of a tablet with a laser beam of a predetermined strength and diameter. A color sensor verifies the side to be drilled. A vision system then verifies the presence of the drill hole. If the drill hole is present, the tablet is passed on as acceptable. ...
-
Product Capsules/Tablets counting machine
The system within high speed to capsules/tablets counting system,can meet GMP standard for pharmaceutical industry.The Max.speed can up to 200bottles/min.The feeding system use serve motor and sensor tracking.And within view vision reject system to reject the no good products.
-
Product ENGINE ASSEMBLY AND CONTROL PLATFORM
This platform allows the assembling of cosmetic engines with in process controls .
-
Product Roll-on
Sopac médical offers a wide range of services which includes applicator and nozzle. Contact us for more information.
-
Product Injection vials
Our injection vials are mostly used for human and veterinary medicine, analytics and laboratory supply. Please find some more information including the standard sizes on our website: https://pharmaglas.de/injekten.html
-
Product Oral Syrups - Suspension/Dry/Liquid
Pharma Access is an end to end Solution provider for timely execution of Complete Turnkey Projects in the field of Pharmaceuticals, Vaccines and Biotechnology in all pharmaceutical forms viz-a-viz;Inhalation (MDIs and DPIs) Sterile injectable (SVP, MVP and LVP) and non-injectables Oral Soli...
-
Product Large volume parenteral manufacturing (LVP)
Weiler engineering provides wide range of pharmaceutical and healthcare applications which includes large volume parenteral manufacturing (LVP). It belongs to injectable products/SVP /LVP products category. It is a typically injectable products designed for intravenous delivery applications. Contact us for...
-
Product Solmed medituub cler-s
Renolit hansen packaging tech.(bj) ltd offers a wide range of products which includes solmed medituub cler-s. Features: it has cardiovascular applications e.g. During open heart surgery. It ensures superior blood contact properties. Both ends of the roll of tubing are closed by high frequency sealing to pr...
-
Product Soliboard folding cartons
With more than 2,5 billion folding cartons produced each year for the Pharmaceuticals / Personal care sector, LGR Packaging has a perfect knowledge of the product and offers a wide range of solutions, regarding design, printing, finishing, functionalities.
• DESIGN: simple co...
-
Product Fold Pack pails
Square Fold Pack pails increase pallet efficiency and reduce the costs of empty storage and transport. Hassle-free and fast opening and closing thanks to a brand new closure type.
Plastic High Performance Packaging by CurTec is clean, safe, and secure and offers maximum protection to active ingred...
-
Product Ampacet ProVital+ White 209 masterbatch for medical applications
Complete consistency of performance, traceability and control of raw materials used for fabrication of the ProVital + range is crucial for manufacturers of medical devices, primary packaging and in-vitro diagnostics equipment.
Produced under strict hygienic manufacturing conditions and...
-
Product Device Assembly and Packaging
The injectable drug market is rapidly evolving and includes an array of delivery devices from autoinjectors to pens and other intuitive formats. We provide partnership for drug owners driven by the top-quality, complex considerations and flexibility expected. These services include assembly process des...
-
Product AL-AL strip foil (easy tear film)
Sichuan Hui Li Industry Co. Ltd. offers a wide range of soft package composite film products which includes al-al strip foil (easy tear film). Contact us for more information.
-
Product Biocompatibility & Toxicology Testing
Nelson Labs offers a full trifecta of services to meet the requirements of ISO 10993 for Extractables & Leachables, Biocompatibility and Toxicological Assessments of medical devices. Biocompatibility testing is very common in the medical device industry. However, with 24 possible categories, each with ...
-
Product Liquid stick-packs
Pharmatis manufactures pharmaceutical products which includes liquid stick-packs. It is avilable in liquid forms: aqueous and hydri-alcoholic solutions, suspensions, emulsions, gels. Packaging: stick-packs pharmatis has 2 lines and 10 track fully automated line, stick-pack content: 3 ml to 15 ml, width: 25...
-
Product Dental cartridges
Kapoor Glass provides wide range of pharmaceutical products which includes Dental cartridges. These are produced using speciality sourced tubing produced with tightened tolerances. The product complies with the highest quality standards. Ceramic printing is also available.. Contact us for more information.
-
Product eMDI Connected Metered-Dose Inhaler
H&T Presspart has developed the first market-ready, fully embedded and connected metered-dose inhaler (eMDITM). By tracking and recording data on the use of medications, and sharing it with patients and physicians via a mobile or web app, the eMDI enables pharmaceutical manufacturers to bring the best poss...
-
Product 10ml Crimp-on Bottle
BONA Pharma offers a wide range of products which includes 10ml HDPE crimp-on bottle, round shape, different color available, injection blow molding with stable quality and lower cost. It belongs to bottles for liquids category.dispenser sub type: bottle - medicine. Contact us for more information.
-
Product Pharmaceutical Packagings
Combi Seal Euro Cap Flip Off LDPE,Pharma Grade Moulded Glass Injection Vial, Type I/Ii/Iii Rubber Stopper Plunger Prefilled Syringe PP,Pharma Grade
-
Product ClearJect®
Prefilled Syringes (Prefill Syringes, Prefillable Syringes) are indispensable in the medical field. Since the drug solution is prepared in the syringe, it is highly excellent in handiness, safety and sterility. Followings are our prefilled syringes.
-
Product cleanpeak Beta-Port Bag
- For entry of components (Stoppers, tools, pumps, etc) into isolators, RABS in the cleanroom
- Single-use product for high grade clean environment
- Waterproof, b...
-
Product SYFPAC® TWIN - BLOW FILL SEAL MACHINERY
SYFPAC® TWIN is Brevetti Angela double-mould machine for aseptic filling of Small Volume Parenterals with the Blow Fill Seal (BFS) technology.It is designed for high production of plastic containers from 0.2ml to 20ml in the shape of vials and ampoules. Starting from the polymer granule, the container is f...
-
Product Folding Boxes
• Folded cardboard boxes with gluing in 1, 2, and 3 points • Straight and folded leaflets Raw material:
• GC, GD, GT cardboard of weight between 230 and 450 g/sqm • Offset paper of 40, 45, 60 g/sqm
-
Product Drug Product & Finished Goods
End-to-endservices designedfor patient safetyand centricity while offering contracting convenience, cos tefficiencies and time savings. Our globally integrated drugproduct (DP) and finished goods (FG) services offer agileand flexible solutions for clinical to commercial products. Our DP capabilities includ...
-
Product Oral, Vaginal, Rectal
We offer millimeter solutions in medicine applicators and dispensers, being today a global reference for quality and precision in this segment.
Our systems aim to be used for oral, vaginal and rectal treatments such as Oral infections, Vaginal infections, and Rectal diseases.

-
Product Aerosol Cans
A new generation of innovative, alternative-shaped aluminium aerosol cans are already a new standard which fits the individual needs of our customers. Rimmed, shaped, transfer flat shoulder, bossed cans are all possible for Perfektup Aerosol Business Unit to produce. Perfektup Aerosol has been producing ri...
-
Product Injectables (Aseptic Filling)
Specialist in filling and packaging SVP (small volume parenteral) in prefilled syringes and vials. Our injectable plants export over 50 countries worldwide.
- 5 high speed lines for syringes (aseptic filling)- 3 high speed lines for vials (aseptic filling)
- Terminal sterilization
- Fully equipped...
Pharmaceutical Packaging
Packaging is an essential part of every manufacturing and production process. Pharmaceutical packaging involves the provision of an appealing presentation, portable containment, reliable protection, convenience, and identification information for a product for storage, distribution, and sales. This containment is essential for preserving drugs and drug products during storage, transportation, and display, till the drug or drug product is consumed or administered.
Pharmaceutical packaging must be designed in such a way that it protects the drug or drug products from external factors such as temperature, sunlight, micro-organisms, etc., and maintains its stability. In the healthcare sector and the pharmaceutical industry, pharma packaging plays a significant role in the safe procurement and administration of pharmaceutical products. Here, we have carefully examined the types, categories, the market size of pharmaceutical packaging, and its importance to the pharmaceutical industry.
Categories of pharmaceutical packaging
In the healthcare and pharmaceutical sectors, close attention is given to pharmaceutical packaging as it is used to complete the medical prescription of various drugs for identification, storage, and consumption. There are three categories of pharmaceutical packaging used for every pharmaceutical product regardless of the dosage form. These categories include primary, secondary, and tertiary packaging.
Primary packaging consists of materials that have direct contact with the drug or pharmaceutical product packaged. This is the material surrounding the drug. The materials must be inert, safe, and capable of protecting the product from external factors such as moisture, climatic changes, biological contaminations, etc. Some primary pharmaceutical packaging types include sachet packaging, bottles, glass vial, injectables, blister pack, ampoules, etc.
Secondary packaging refers to boxes or cartons where the package is kept. This usually contains information and directions of the pharmaceutical product. Tertiary packaging refers to the last stage or level of packaging carried out to aid transportation, shipping, and wholesale storage. Tertiary packaging primarily protects the product, primary and secondary packaging from external factors during transit.
What are the types of pharmaceutical dosage form containers?
There are different types of pharmaceutical dosage form containers, and these containers are classified based on the dosage form ranging from solid to gas formulations. Based on dosage forms, there are solid dosage form containers, liquid dosage form containers, and gas dosage form containers. Solid dosage form containers consist of tamper-resistant packaging such as Bottle seals, strip packages, blister packages, sealed tubes, breakable caps, tape seals, shrink seals and bands, film wrappers, foil paper, child resistant packaging, etc. Liquid dosage form containers consist of glass bottles, ampoules, cartridges, vials, syringes, plastic container, etc.
These containers can either be single-dose or multiple-dose containers, airtight containers, well-closed containers, or light-resistant, depending on the drug packaged. Gas dosage form containers include sprays, vaporizers, prefillable inhalers, aerosols, and nebulizers—the majority of these containers consisting of airtight plastic or glass container with spray pumps. For semi-solids, plastic containers, pressurized packages, and metallic collapsible tubes are used.
Other dosage form containers such as liposome packaging include hermetically sealed borosilicate glass ampoules and narrow and wide-mouth borosilicate glass bottles.
What is the global market size of pharmaceutical packaging?
The size of the global pharmaceutical packaging market plays a valuable role in the global pharmaceutical industry market. The increasing need for drugs has positively affected the growth of the global pharmaceutical market size. Based on Precedence research, the global market size in 2019 was valued at US $98.83 billion.
In the following forecast period from 2020 – 2027, it is expected to experience a compound annual growth rate (CAGR) of 8.6%, surpassing the value of US $191.21 billion by 2027. The growth of the global pharmaceuticals packaging market size has been contributed to by various key factors such as the increasing demand for blister packaging, patient-oriented drug, increasing aged population, rising health awareness, etc. Pharmaceutical packaging and contract packaging companies have significantly propelled the growth of the pharmaceutical industry.
In 2019, the global market was led by North America, with a value share of about 38% due to the presence of leading pharmaceutical packaging companies within that region. Also, the primary pharmaceutical packaging segment had an estimated value share of 75%, and this is expected to continue over the forecast period. The Asia Pacific is expected to witness a rapid growth rate over the forecast of 12% due to the rapid increase in population size.
How many types of pharmaceutical packaging are there?
Each of the categories/levels of packaging has different types, and the nature of these packages are dependent on the drug dosage form – solid, semi-solid, liquid, and gas. There are numerous types of pharmaceutical packaging based on three different categories. A few of them include:
-blister packs
-vial packaging
-ampoules
-prefilled syringes
-latitudes
-plastic bottle
-glass bottle
-cartons and boxes
-plastic pouches
-strip package
-fin sealed package
-shrink seals
Materials used in pharmaceutical packaging
Just as the types of packaging vary, the raw material used in the production of these packaging containments also vary. Various pharmaceutical packaging companies produce different packaging types, and they are made from other raw materials, organic and inorganic. The choice of pharmaceutical packaging material used for the production of packaging containers is usually dependent on the type of drug to be packaged and specific patient needs. In some cases, these materials can be referred to as primary packaging when they directly contact the drug formulation. Some examples of materials used include glass, plastics, elastomeric material such as rubber, etc.
What are the various packaging materials used in the pharmaceutical industries?
Pharmaceutical companies manufacture drug packaging using various raw materials. Due to technology and research advancement, the range of material options continues to increase. As new materials are discovered and chosen for use, factors such as cost, convenience, dosage form, degree of protection required, sterilization methods, drug-material compatibility, and filling methods are considered. Each material considered or chosen for packaging must be a sustainable material to improve safety and drug usage.
Given the different types of dosage forms, stability, and effect of drugs, a wide range of pharmaceutical packaging materials are used, such as glass, rubber, plastics, metals, paper, films, aluminium foil, and laminates, and fibrous material.
What are the main packaging materials used in pharmaceutical industries?
Although there are various packaging materials, there are a few major ones used by almost all pharmaceutical companies and for most packaging. Glass and plastic are some of the most commonly used materials in drug packaging.
Glass has been used for drug packaging for several years now due to the numerous benefits it offers. Glasses (glass bottles) are transparent, economical, have adequate protection power, can be labeled easily, come in various shapes and sizes, and durable. They are thought to possess high protection power due to their inert composition, which eliminates or reduces the chances of drug-material interactions. The most commonly used glass packaging is amber glass because it offers more protection from U.V. rays than clear glass.
Plastics are also commonly used because of their versatility, flexibility, ease of use, durability, and light weightiness. However, the downside to its use is the possibility of drug-material interactions through the transfer of leachable. Some types of plastics used include polypropylene (P.P.), polyethylene terephthalate (PET), high-density polyethylene (HDPE), and several others.
What are the organic packaging materials available that are used in the pharmaceutical industries?
Pharma packaging companies are progressing to the use of eco-friendly, organic, and biodegradable materials for packaging production. This innovation is considered more appealing to consumers, environment-friendly, and a plus for brand reputation.
Organic raw materials used for packaging production can be derived from plants, animals, agricultural feedstocks waste, and marine food processing industry waste. The use of organic and biodegradable materials helps to maintain a safe environment and atmosphere. Some popularly used organic packaging materials include paper, cork, starch, chitin, cellulose, gelatin, collagen, gluten, soy, keratin, etc.
Accountability of pharmaceutical packaging manufacturers
The development, manufacturing, and production process of drugs and drug products are carefully monitored and inspected to ensure that regulatory policies are duly recognized and followed. Pharmaceutical packaging manufacturers are held accountable by regulatory organizations such as the World Health Organization (WHO) and the Food and Drug Administration Agency (FDA).
Accountability of these packaging companies ensures that the raw materials used for the production of packaging and the final products do not affect the stability, safety, and effectiveness of the drug or drug product.
What are the risk factors in the packaging of pharmaceutical products?
Although pharmaceutical packaging is essential and the benefits of pharmaceutical packaging are enormous, there are various risks associated with packaging. With every step of the manufacturing process comes a risk to the drug's safety and integrity. In addition to consumer safety, drug stability, drug delivery, and integrity, other factors such as brand name, reputation, and profitability face various risks associated with packaging.
Risk factors associated with package design and production include wrong labeling, incorrect or incomplete product information, material-product interaction (containment-drug reaction), contamination, poor seal integrity, supply-chain interference, destruction of products as a result of mishandling, incorrect product count or fill-level, etc.
Other factors influence these risks, and they can become sources of paramount concern when these factors come into play. Some of these factors include a non-sterile processing environment, faulty machines and equipment, damaged raw materials, incompetent staff, and many others.
These risk factors put the company's reputation and profits at risk and the health of patients (consumers).
Who regulates the pharmaceutical industry?
Given the importance of pharma packaging and its effect on drugs, drug products, and consumer safety, strict regulatory guidelines and policies are put in place for pharmaceutical manufacturing companies. These regulatory guidelines and procedures are put in place by regulatory agencies and organizations such as the U.S. Food and Drug Administration (FDA) to ensure good manufacturing practice (GMP) in each pharma company. They ensure that pharma companies provide standard packaging systems, through the use of standard pharmaceutical packaging equipment (packaging machine) or medical devices.
Although the regulatory agencies vary by country, they are global pharmacopeia standards set by the World Health Organization (WHO) which are globally acceptable.
The standards and policies created are implemented to ensure material-drug compatibility, prevent contamination, preserve drug integrity, maintain stability, and ensure patient safety. Pharmaceutical packaging standards deal with production location and its sterility, packaging labels, and packaging materials. Some guidelines that have been set in place across regions globally include Anti-tampering regulations issued by FDA, Labelling regulations (Fair Packaging and Labelling Act 1967), and others.
Pharmaceutical and biopharmaceutical supply chain expertise
The pharmaceutical supply chain consists of several government agencies, research organizations, clinics, hospitals, drug manufacturers and distributors, pharmacies, retailers, and regulatory agencies such as the FDA. This chain is responsible for manufacturing drugs, distribution of prescription and OTC drugs, biologics, and generics, and others.
In some cases, insurance companies, HMOs, and GPOs form part of the supply chain or packaging line, which further complicates it.
Due to the complexity of the supply chain, the supply chain's management and regulation may pose a challenge. However, several trusted partners and companies are well-versed and experienced in the management of the pharmaceutical and biopharmaceutical supply chain; a notable example includes Novartis.
Finding the right partners in a supply chain can be effectively carried out when due research and communication are conducted on the key players of the supply chain.
Latest trends in pharmaceutical packaging
Over time old trends have passed and given way to new ones. While the pharmaceutical packaging industry continues to evolve, various trends arise which are shaping and changing the future of the pharmaceutical packaging industry. These latest trends have come to modernize and transform the global pharmaceutical industry for the benefit of the manufacturers and the consumers.
These new trends have actively contributed to the growth of the global pharmaceutical market size in recent years. However, they are also projected to contribute a large percentage of the worldwide market size growth in the nearest future. These key trends have been identified based on collected data and insights gathered by pharmaceutical industry experts.
What are the new trends in pharmaceutical packaging?
The Global pharmaceutical industry has changed rapidly over the years and is still evolving. Due to the increasing population, demand for drugs, and many other factors, the need for new and modernized drugs is on the rise. As a result, there is need for a new pharmaceutical packaging solution and design to accompany the production of these new drugs.
Accompanying new drug developments are recent pharmaceutical packaging trends in the pharmaceutical manufacturing organizations to ensure consumer safety and convenience, manufacturer's profitability, and boost brand reputation.
Some of the newest trends impacting pharmaceutical packaging include Eco-friendly packaging, innovative packaging automation and machinery, sustainability, smart packaging, minute production lots, patient engagement through increased accessibility, and ease of production. Newer innovations directed at meeting patients' needs, ensuring the company's security needs, and maintaining quality include unit dose vials, child-resistant packs, prefilled syringes, and blow fill seal vials (BFS), etc., have also impacted pharmaceutical packaging.
What is new in the world of smart packaging?
Technology is rapidly evolving, and every sector is making efforts to step into the future early, the pharmaceutical industry inclusive. Smart packaging is an outstanding innovation in the pharma industry. With the invention of new pharmaceutical and biopharmaceutical products, pharma companies need to provide new means of storage and transport of drugs and drug products to maintain their integrity.
Wrongly or poorly packaged drugs can pose numerous health risks to the consumer. Also, damage to good packaging leaves drugs and drug products liable to damage and can even pose devastating health risks to the consumers. So, this is where smart packaging comes to play. Smart packaging provides tools that can help preserve pharmaceutical containers and improve consumer cooperation and use. Some new intelligent packaging trends include the use of time-measured indicators, bar code, temperature-oriented indicators, ultra-thin ICS, Near-Field Communication (NFC) tags, and chemicals or biosensors to indicate the safety of the drug.
These packaging innovations drive the brand or pharmaceutical company's success, improve consumers’ health, prevent drug counterfeiting and consumption of these counterfeit or damaged drugs and drug products.
Who are the leading companies operating in the global pharmaceutical packaging market?
Considering the importance of quality packaging and processing, it is no surprise that there is a rising competition between pharmaceutical processing and packaging companies within the pharmaceutical industry. These key players have actively contributed to the growth of the global pharmaceutical packaging market and the pharmaceutical industry.
These companies are considered to be leading players based on their yearly generated revenue, mergers and acquisitions, and their ability to stay at the top of key trends per time. Some of the leading packagers in the global pharmaceutical packaging market include Amcor Limited, Aptar Group Inc., Catalent, Bilcare Inc., ACIC Pharmaceuticals Inc., Marchesini Group S.p.A., COESIA S.p.A., IDEX Corporation, Kȍrber AG, GEA Group, Trucking Technology Limited, Berry Global Inc., Gerresheimer AG, Scott AG, Berry Global Inc., West Pharmaceutical Services, Inc., WestRock Company, and many others.
The importance of the right type of pharmaceutical packaging cannot be overemphasized. This applies not only to pharmaceutical formulations but other products in different industries. It is important to note that a specific packaging material may not be suitable for two different drugs because of its components.
Given the importance of pharmaceutical packaging, it is no surprise that the pharmaceutical packaging and processing company is a significant pharmaceutical industry sector. They largely influence the market growth of pharmaceutical industry.
References
https://blog.drupa.com/de/pharmaceutical-industry-benefit-intelligent-packaging/
https://www.originltd.com/useful-resources/product-information/types-of-pharmaceutical-packaging/
http://eprints.ugd.edu.mk/19277/3/10.%20FORMULATION%20AND%20PACKAGING.pptx
https://www.pharmatutor.org/articles/the-pharmaceutical-packaging-article?amp
https://www.pharmaguideline.com/2018/03/packaging-of-pharmaceutical-products.html?m=1
https://www.idosi.org/mejsr/mejsr12(5)12/16.pdf
References
Upcoming Events
-
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025 -
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025 -
CPHI & PMEC China 2025
Shanghai New International Expo Center
24 Jun 2025 - 26 Jun 2025
Pharmaceutical Industry Webinars
-
Webinar An Untapped Market in Gender Inclusivity and Equity
-
19th March 2025
-
3pm GMT /4pm CET
-
-
Webinar Pathway to $10/g Biologics Production: Reshaping the Biomanufacturing Landscape
-
18th February 2025
-
3pm BST /4pm CET
-
-
Webinar Leveraging Real-Time Clinical Data to Deliver Certainty in Solubility Enhancement and Modified Release Development
-
12th December 2024
-
3pm BST/ 4pm CET
-
-
Webinar The CPHI Sustainability Collective: A New Initiative to Support a Sustainable Pharma Value Chain
-
10th December 2024
-
3pm BST /4pm CET
-
-
Webinar Key to Success: A CDMO's Pathway to Biologics Excellence
-
5th November 2024
-
3pm BST/ 4pm CET
-
-
Webinar The Changing Dynamics of Global API Manufacturing
-
17th September 2024
-
3pm BST/ 4pm CET
-
-
Webinar Shaping the Future of Italy’s Pharma Market: Trends and Opportunities
-
4th September 2024
-
4pm CET/ 10am EST
-
-
Webinar Fragment-Based Oligonucleotide and Oligopeptide Synthesis
-
30th Jul 2023
-
4pm CET / 10am EST
-
-
Webinar GMP Rationale for Sterile High-Potency/Toxic Pharmaceuticals
-
18th June 2024
-
4pm CET / 10am EST
-
-
Webinar Unlocking Opportunities in the Growing Pharma Landscape of The Middle East
-
5th June 2024
-
3pm CET / 9am EST
-
-
Webinar Exploring Technological Trends in the Future of Pharmaceutical Manufacturing
-
23rd May 2024
-
4pm CET / 10am EST
-
-
Webinar Achieving Manufacturing Excellence Through Digital Transformation
-
16th April 2024
-
4pm CET / 10am EST
-
-
Webinar Made in Africa: What’s Driving Pharma Manufacturing
-
28th March 2024
-
4pm CET / 10am EST
-
-
Webinar Case Study: Risk Management for Annex 1 Sterile Production EMS
-
28th February 2024
-
4pm CET / 10am EST
-
-
Webinar Innovative Strategies for B2B Pharma Marketeers: Driving Value through Content
-
20th February 2024
-
4pm CET / 10am EST
-
-
Webinar Revolutionizing Pharma: Data and AI Unleashed
-
18th January 2024
-
4pm CET / 10am EST
-
-
Webinar Optimal Temperature: Elevating Biologics Cold Chain Excellence
-
16th January 2024
-
4pm CET / 10am EST
-
-
Webinar Market Outlook – The Biggest Pharma Trends of 2024
-
12th December 2023
-
4pm CET / 10am EST
-
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance














































































-comp295228.jpg)









































-comp241444.jpg)













































































.png)





.png)
.png)
.png)
.png)




.png)





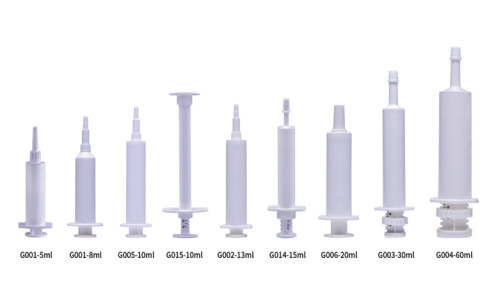




















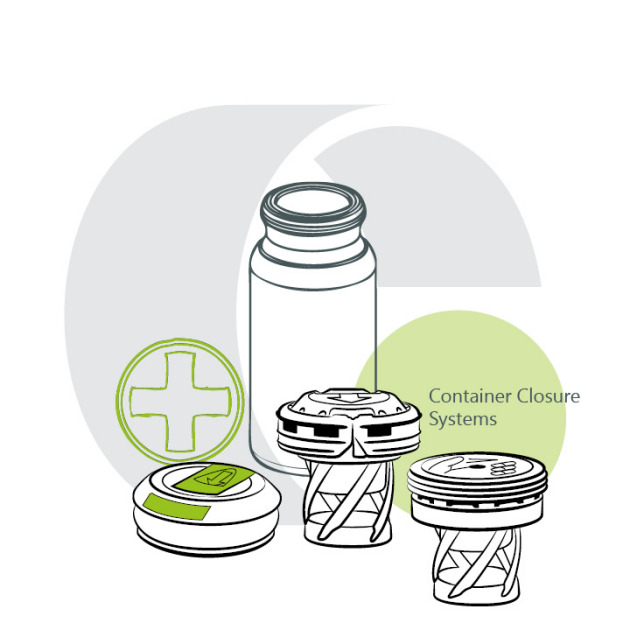
















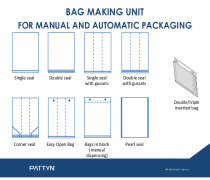






.png)

















.png)










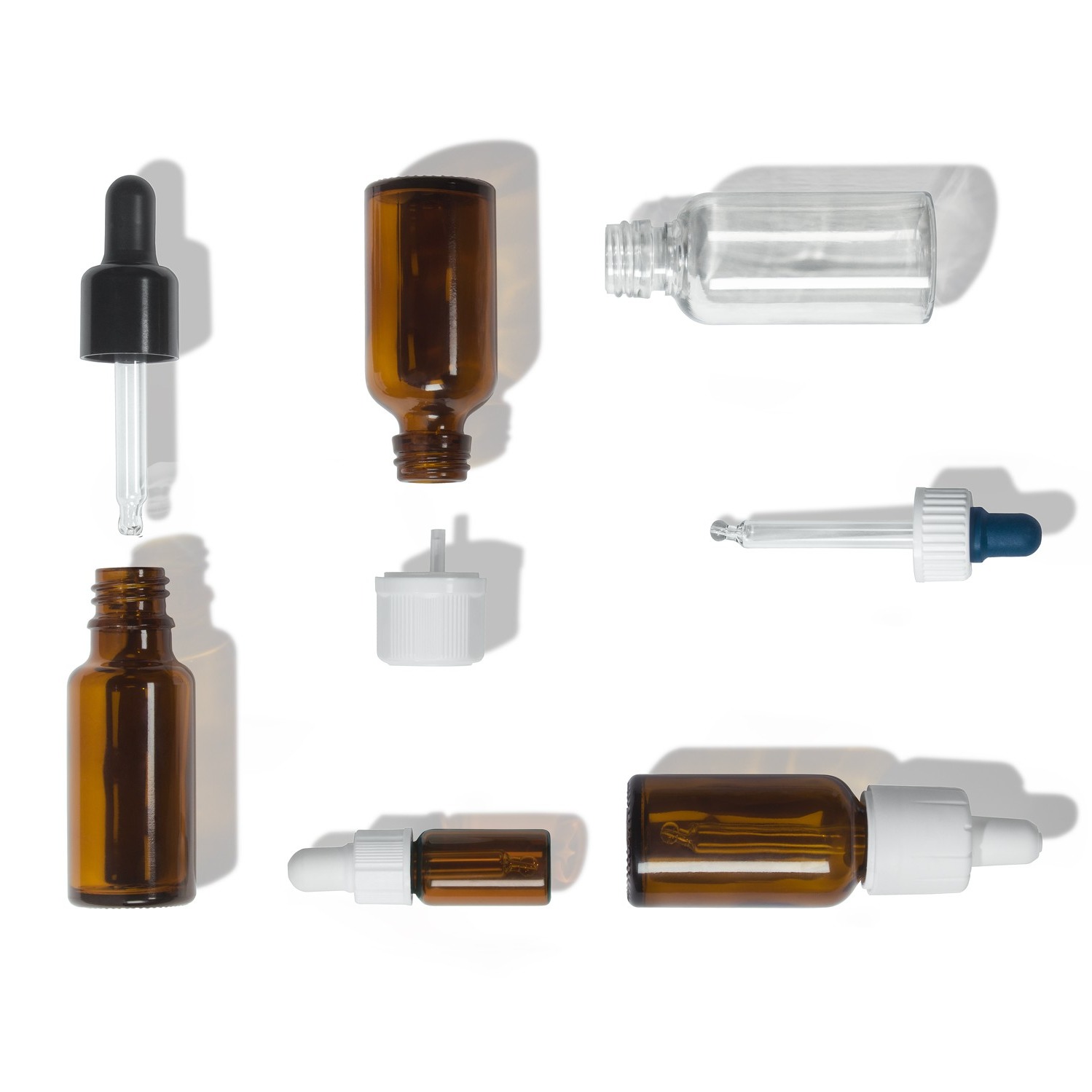














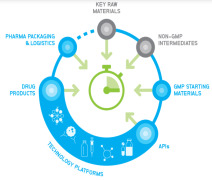










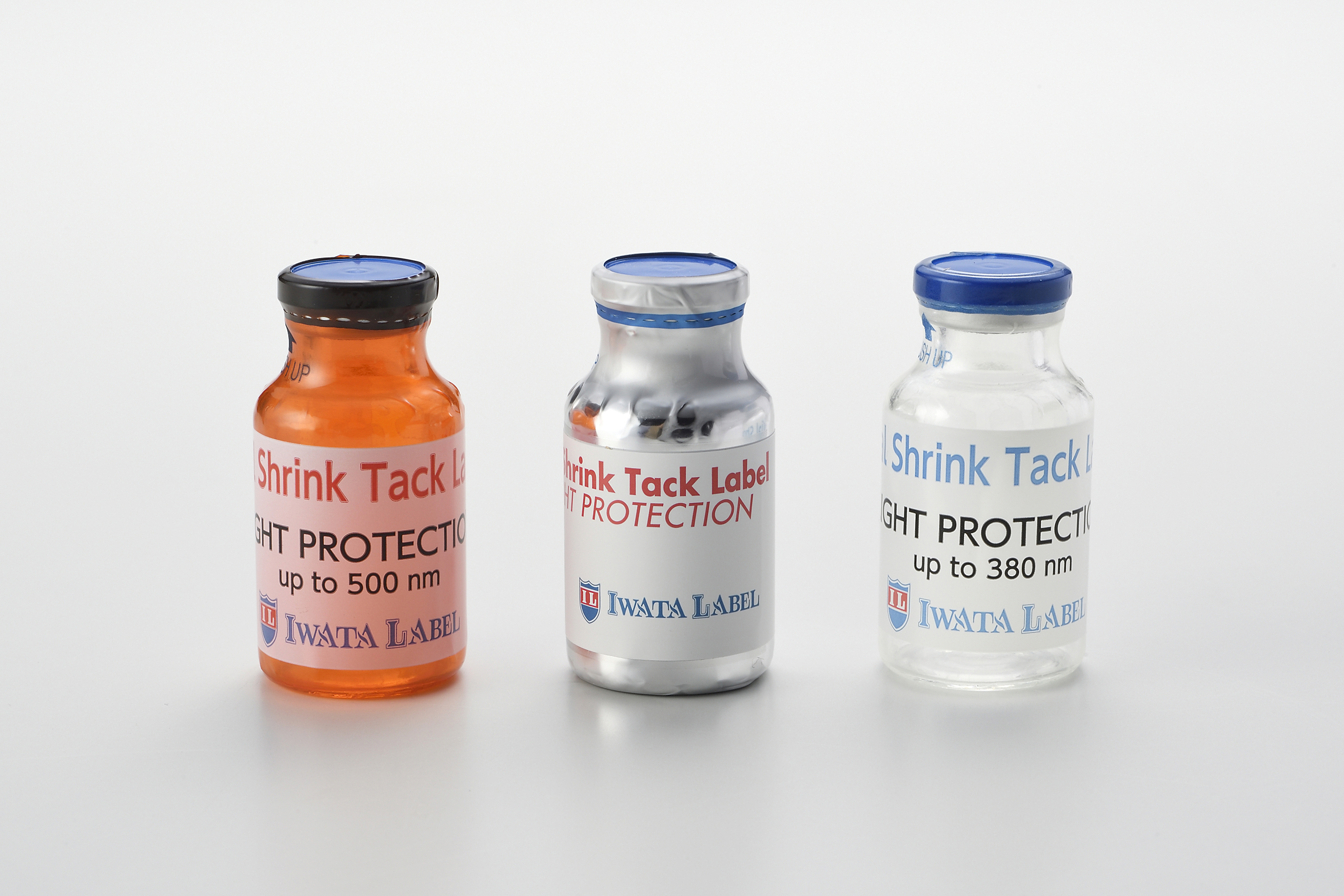











.png)












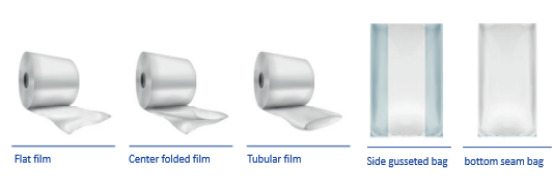
















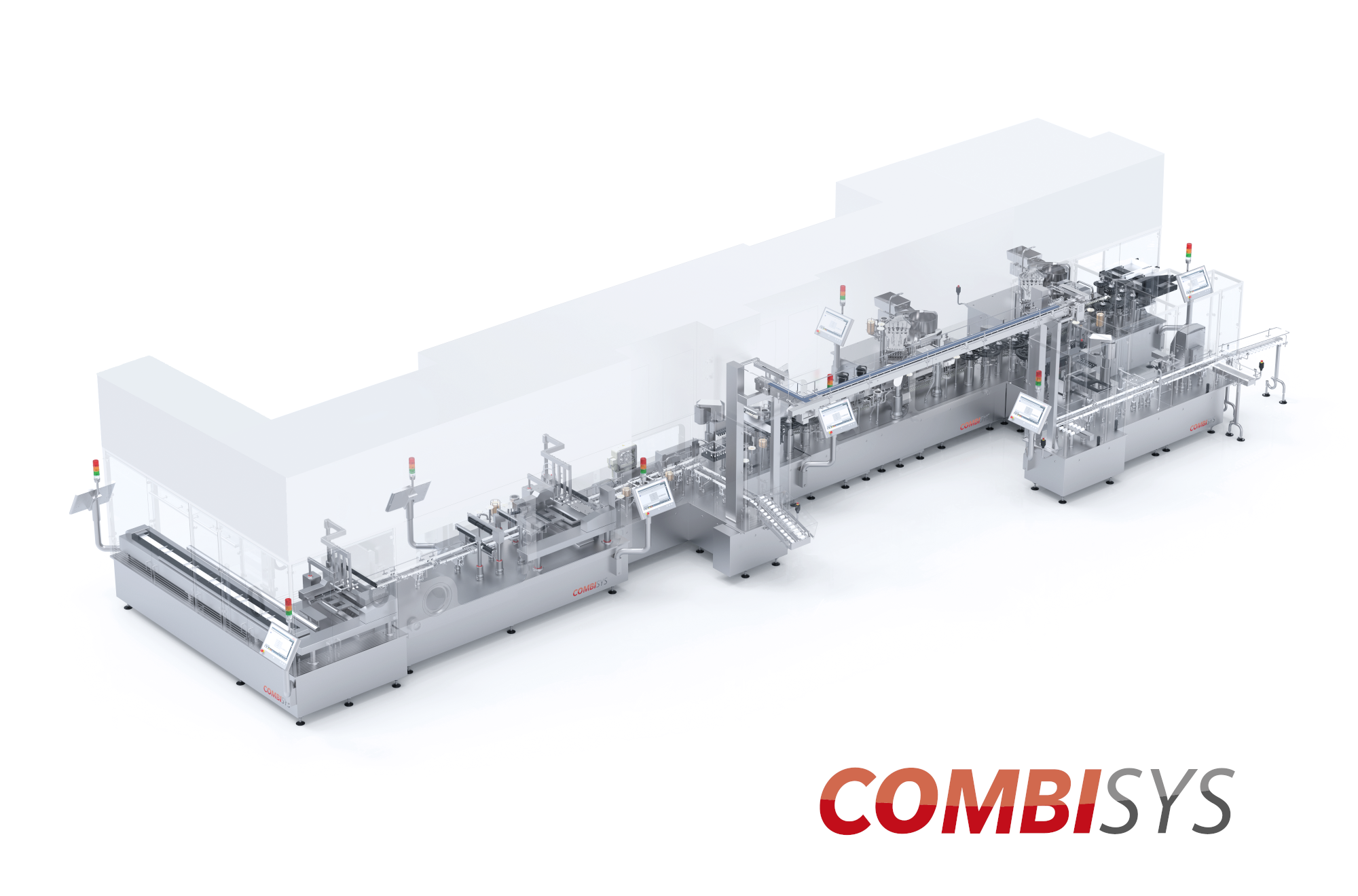





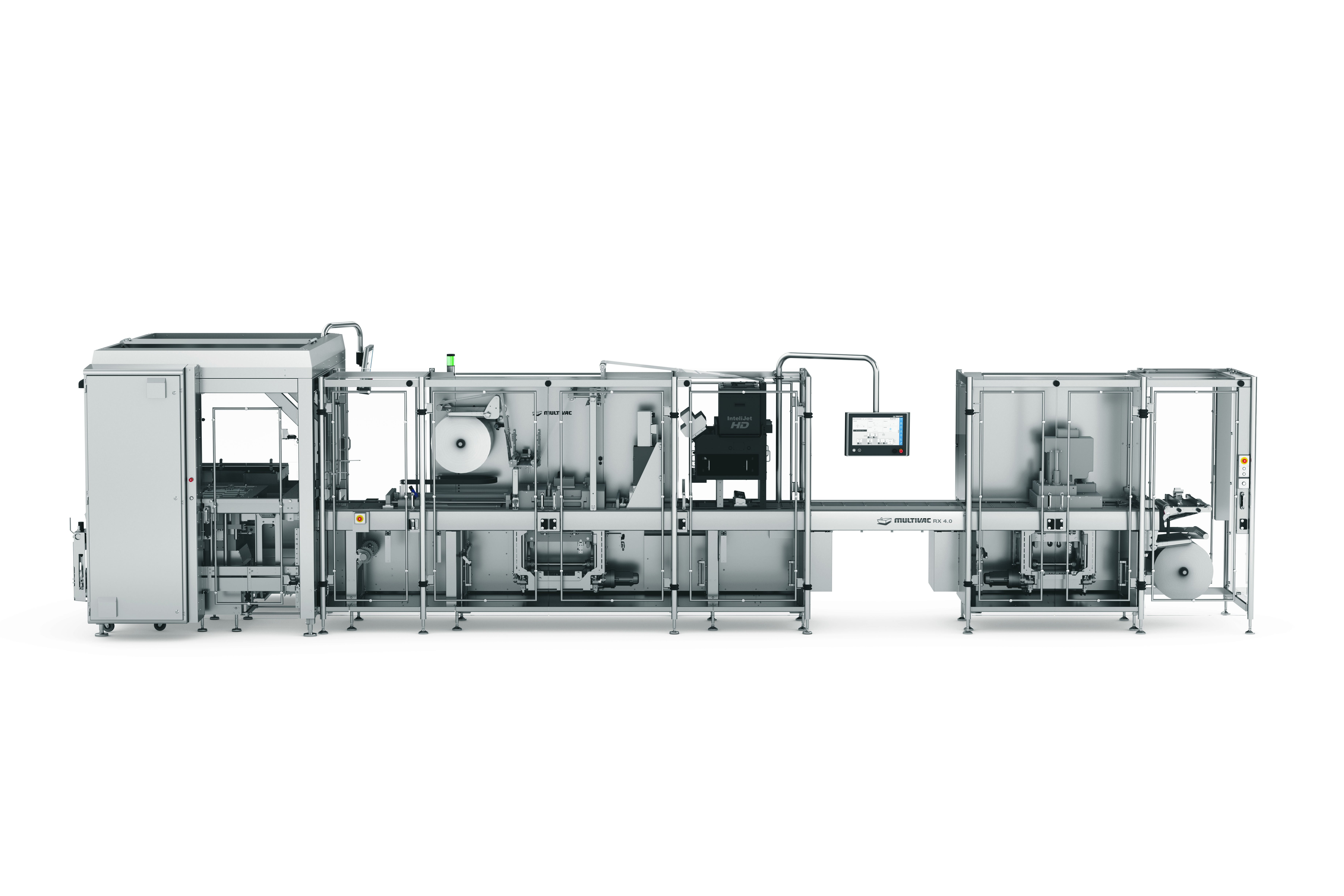



















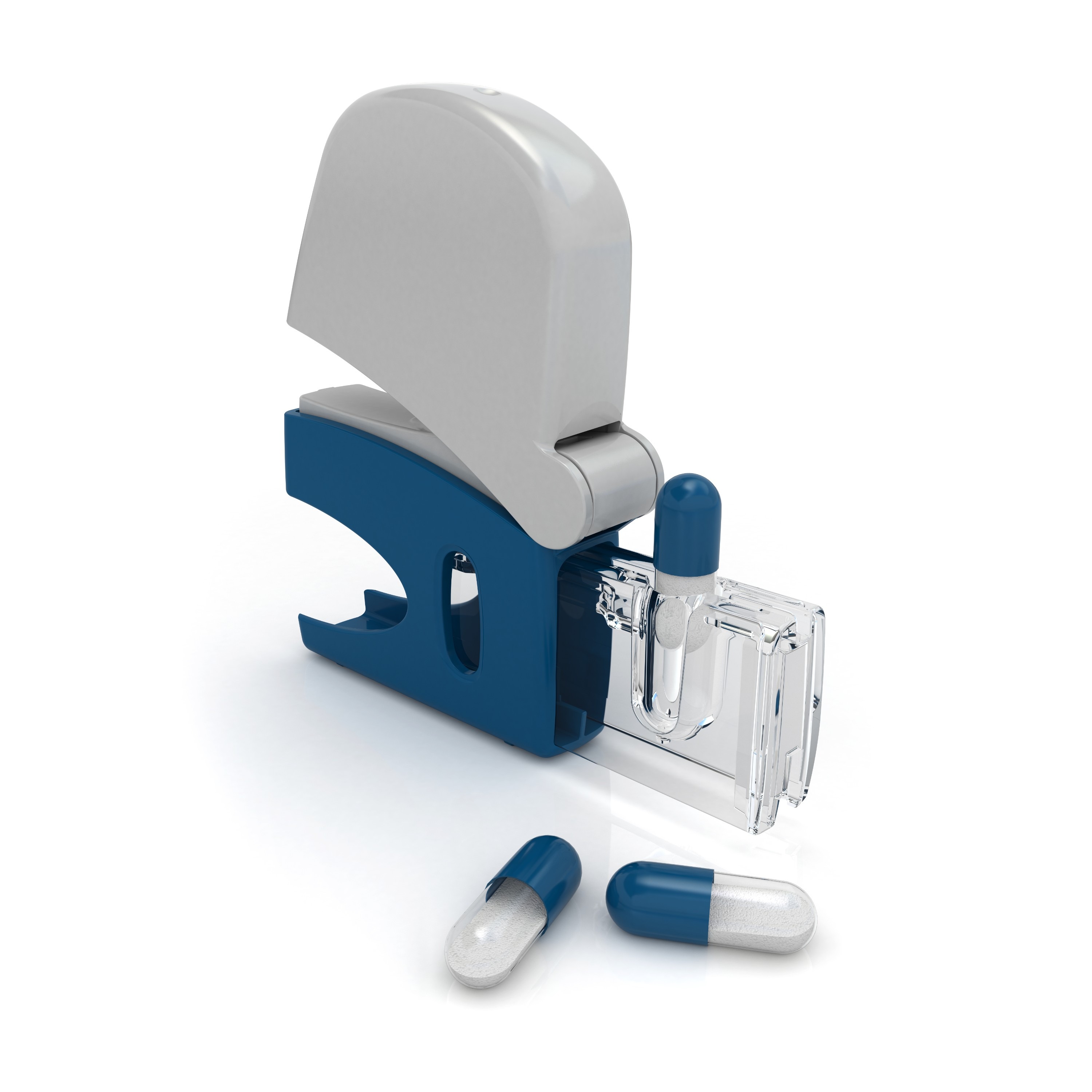

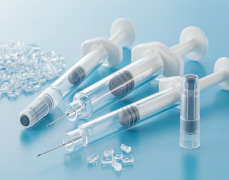

.png)



.png)







.png)

.png)



.jpg)
