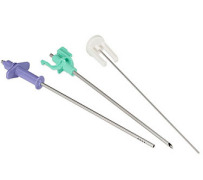
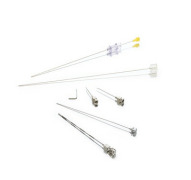
End-to-end contract manufacturing makes it simple

Product Description
Tegra Medical

-
US
-
2022On CPHI since
-
2Certificates
-
1000 - 4999Employees
Company types
Primary activities
Categories
Tegra Medical

-
US
-
2022On CPHI since
-
2Certificates
-
1000 - 4999Employees
Company types
Primary activities
More Products from Tegra Medical (3)
-
Product Manufacturing a variety of drug delivery devices
Drug delivery devices need to be simple for patients and practitioners to use, but manufacturing them can be complex. Fortunately, manufacturing complex devices and components is Tegra Medical’s specialty and takes advantage of our expertise in combining non-traditional and common manufacturing techno... -
Product Product development and prototyping services
Tegra Medical will help get your new drug delivery devices to the marketplace. Our GENESIS Tech Center® engineers are experts at refining your ingenious designs with Design for Manufacturing (DFM) to reduce cost, increase quality and shorten the development cycle. Partnering with you, we’ll ensur... -
Product Keep your sharp drug delivery devices sharp
Tegra Medical is renown in the industry for making sharp tips and keeping them sharp throughout the entire manufacturing process.
The metal “business end” of a device can be very delicate. The soft metal is good for one or two cuts. So, sharpness is critical. At Tegra Medical...
Tegra Medical resources (5)
-
Video Introduction to Tegra Medical
Mike Treleaven, Senior Vice President Engineering provides an overview of Tegra Medical and its renowned expertise in keeping sharp parts sharp. -
Brochure Not your average manufacturing partner
An article by Senior Vice President, Engineering, Mike Treleaven for Medical Device Developments, in which he discusses how Tegra Medical works with clients from the early stages through the entire manufacturing process, simplifying the supply chain and guaranteeing efficiency. -
Brochure The perfect mould is a work of art
As this article discusses, injection-moulded medical devices and components may look simple to the untrained eye. But, in fact, they can have extremely tight tolerances and require moulds built with the precision of a Swiss watch. Florian Beck, who heads the development team at Tegra Medical/Stamm in Hallau, Switzerland, writes about what is involved in mould making in this article published in Medical Device Developments. -
Brochure Soft skills complement hard engineering
In this article published in Medical Device Developments, Dean Petrella, Director of Program Management, discusses the “soft” aspects of Design for Manufacturing (DFM): willingness, trust, and partnerships. Getting close to customers whose devices are in the product development stage sets the tone for successful, long-term relationships. DFM is one of the foundations of our GENESIS Tech Center® product development services. -
Video Design for Manufacturing
Dean Petrella, Director of Program Management presents on how we approach Design for Manufacturing (DFM) to help refine product designs to make them more efficient to manufacture.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance