- Home
- Innovative APIs
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
Innovative APIs
Innovative APIs Companies (177)
Innovative APIs News
-
News Scaling the Industry: CPHI Scale-Up Market interview with YSK Laboratories
For the first time, CPHI Milan hosted the CPHI Start-Up Market, expanding support for emerging and small-sized enterprises in their transition to the next level of growth. In this interview, we spoke with Yuvansh Khokhani, Managing Director of YSK Labo...19 Nov 2024 -
News How to disrupt an industry as big as pharma for the better?
In this interview, hear from Matthew Wise, Head of Data at CCD Partners, on the companies they've been looking into that are offering new and interesting perspectives that have the potential to shake up the pharmaceutical industry, and how they'...25 Oct 2024 -
News CPHI Pharma Awards 2024: Meet the winners from the CPHI Celebration
This year we had a lot to celebrate, the 35th Anniversary of CPHI, and our esteemed award winners, of which we included two additional categories this year, the Future Leader award, and Woman of the Year award.12 Oct 2024 -
News CPHI Milan 2024 - From the Floor
Milan and CPHI welcome you to 2024 CPHI Milan! As we celebrate the 35th edition of our flagship CPHI show, editors Vivian Xie and Lucy Chard bring you the latest from the show floor, conference sessions, and innovative solutions from all exhibitors, at...7 Oct 2024 -
News CPHI Milan Speaker Spotlight: The microbiome and investing in future therapies
In the run-up to CPHI Milan, we sit down with some of the experts and thought-leaders speaking at this year’s conference.19 Sep 2024 -
News What can you expect from CPHI Milan 2024?
CPHI Milan 2024 marks the 35th anniversary of CPHI. The 35th Edition of the show, taking place 8–10 October, promises to be a celebration of everything pharma, covering every sector, and featuring thought leaders and trailblazers shaking up the i...25 Jul 2024 -
News Esteve invests €100 million in new manufacturing plant in Spain
Spain-headquartered CMO Esteve expands its manufacutring capacity in plants in Girona, Spain to increase global API offerings with advanced technological solutions, through a weighty investment of €100 million.9 Jul 2024 -
News Viral marketing for B2B pharma businesses: a CPHI Online case study
Discover how a Chinese chemical manufacturing company went viral on TikTok, and what their viral success means for the future of B2B digital marketing for the wider pharmaceutical industry and supply chain.24 Jun 2024
Innovative APIs Products (376)
-
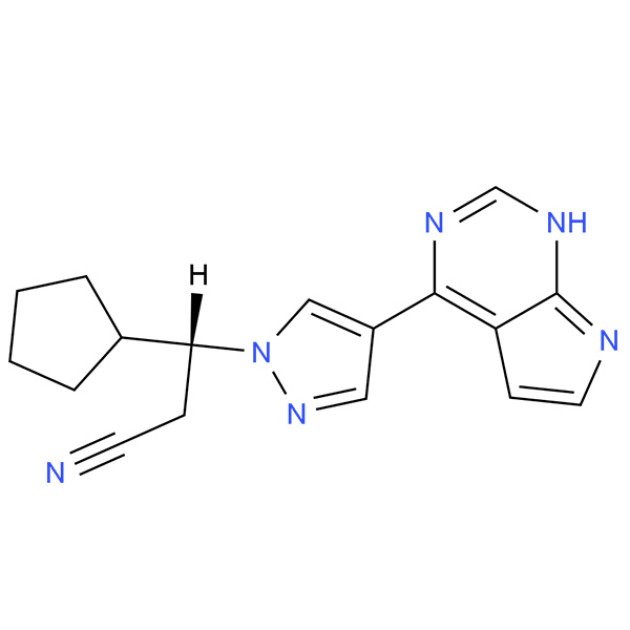
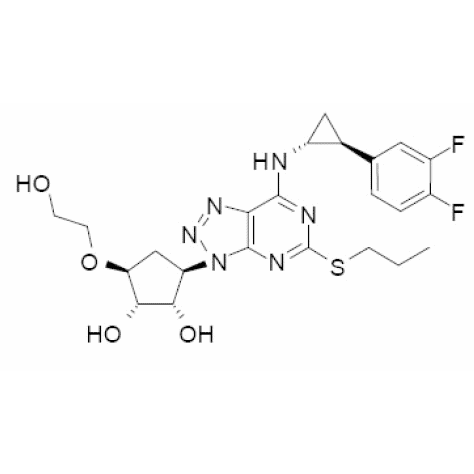
Product Ruxolitinib
Product name: Ruxolitinib phosphate
Synonyms: (R)-3-(4-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile; (betaR)-beta-Cyclopentyl-4-(7H- pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazole-1-propanenitrile; (3R)-3-(4-(7H-pyrrolo[2,3-d]pyriMidin-4-yl)-1H-pyrazo...
-
Product Drug Substance Services
CARBOGEN AMCIS provides comprehensive Drug Substance services, offering expertise in process development, scale-up, and manufacturing of active pharmaceutical ingredients (APIs). With state-of-the-art facilities, the company specializes in high-potency compounds, including cytotoxics, and delivers solu...
-
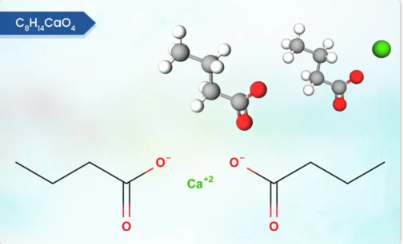
Product Calcium Butyrate
Calcium Butyrate (98%, coated 30, 60 and 90%) is derived from butyric acid, a short-chain fatty acid, and it plays several important roles in promoting the health and performance of animals, particularly in the context of livestock production. It is used in animal nutrition as a feed additive.
-
Product Active Pharmaceutical Ingredients (vitamins, cannabinoids, carotenoids)
Our high-quality active pharmaceutical ingredients undergo the strictest quality assurance protocol, and industry-leading GMP-compliant manufacturing practices, aligned with ICH Q7. With an extensive and well-connected global network, we ensure consistency, reliable and sustainable supply across multiple r...
-
Product Exclusive synthesis
We are experts in process development and manufacturing of exclusive active pharmaceutical ingredients and intermediates.
Your most trusted partner for exclusive synthesis:- More than 150 years experience in process development and production of customized APIs
- Secure and relia...
-
Product VITAMIN D2
Sichuan province yuxin pharmaceutical co. Ltd offers a wide range of products which includes ergocalciferol. It is white crystal powder,smellless,tasteless. Packing: inner packing: aluminium bag, outer packing :aluminium tin. Contact us for more information.
-
Product Ticagrelor
CTX Manufacturer Form - II
O DMF available.
CEP Approved.
CTX Lifesciences respects patent laws and conventions of pharmaceuticals as applicable in different countries.
API/Substances covered by patent are not offered to the countrie...
-
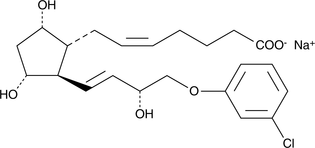
Product CGMP (+)-Cloprostenol (sodium salt)
(+)-Cloprostenol is the active enantiomer of Cloprostenol. Cloprostenol is used in veterinary medicine as a luteolytic agent for the induction of estrus and the treatment of reproductive disorders in cattle, swine, and horses. The ASMF for (+)-Cloprostenol (sodium salt) is on file in several EU member states.
-
Product AB-KOLICARE(R)
AB-KOLICARE(R) is a probiotic formula comprising 2 bacterial strains (B. longum KABPTM 042/CECT 7894 and P. pentosaceus KABPTM 041/CECT 8330) that possess multiple beneficial properties for neonates and toddlers. It is an easy to use, completely natural and safe solution for babies 0+ months old, to treat ...
-
Product Packaging, Quality Control, Storage, Cold Chain
Within the contract development and manufacturing, IDT Biologika GmbH provides a wide range of other pharmaceutical services for our clients products, which include- Labeling & Packaging of vials, prefilled syringes, autoinjectors - Serializiation (Track & Trace) - Quality Systems - Cold Chain up to - 80- ...
-
Product Peptides
We help to accelerate the time-to-market of your peptide APIs and formulations Dr. Reddy's peptide unit has decades of experience in process development and production of peptide APIs and formulations. This experience is brought to bear on a comprehensive offering that integrates a continuum of services ...
-
Product NeoPeptides(TM)
Our NeoPeptide(TM) experts have been associated with the individualised cancer vaccine field for several years and our MHRA registered facility and systems have been established to enable fully GMP compliant manufacture and release of 20-30 GMP peptides within less than three weeks. A bespoke Pharmaceutica...
-
Product Ciprofloxacin Taste Masked Coated Granules (Suspension Grade)
Available in 65% w/w17 Kg Capacity HDPE Container lined with double polythene bags.
-
Product Pemetrexed EVER Pharma
Pemetrexed is an antineoplastic chemotherapy drug. It is used in the treatment of malignant mesothelioma and locally advanced or metastatic nonsquamous non-small cell lung cancer. - Available as a ready to dilute liquid formulation - does not require reconstitution from powder saving time and costs. It can...
-
Product MOXICLAV, Amoxicillin/Clavulanic acid tablets, Powder for Oral suspension, solution for injection
Antibacterial: Penicillin, broad-spectrum & Beta-lactamase inhibitor
-
Product Gobi Gold Daily Balancer(R)
Incorporating PRT(R) Beads and Fusion Technology(R) in Gobi Gold Daily Balancer(R)enabled us to combine the essential fat-soluble and water-soluble nutrientsin ONE capsule simplifying dosing schedule without exceeding therecommended daily dose. We succeeded to combine materials with different properties an...
-
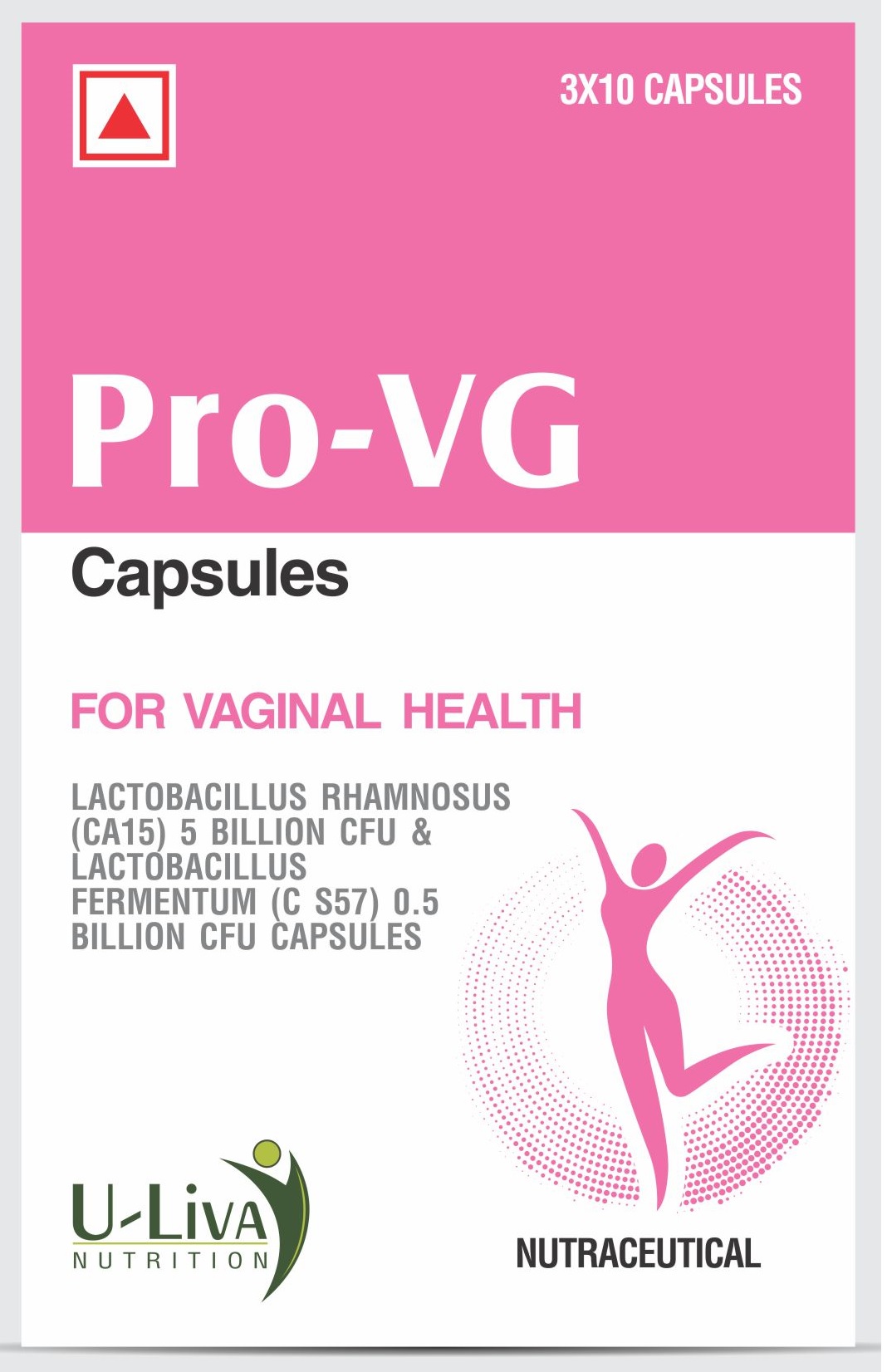
Product PROBIOTIC CAPSULES FOR WOMEN'S HEALTH
PRO-VG CAPSULES FOR WOMEN'S HEALTH ARE DESIGNED, DEVELOPED AND FORMULATED USING CLINICALLY TESTED PROBIOTIC STRAIN TO BE TAKEN ORALY, WHICH HELPS TO FIGHT VAGINAL INFECTION.
-
Product Magnesium oxide
MAGNESIA GmbH provides a wide range of mineral raw materials which includes Magnesium oxides. Contact us for more information.
-
Product Citric Acid Anhydrous (CAA)
Jungbunzlauer offers Citric Acid Anhydrous (CAA) for pharmaceutical use holding a CEP, a Type II US DMF and a GMP certificate. This unique quality standard helps us to provide optimal product solutions for the pharmaceutical industry. CAA is used in a variety of applications, mostly as excipient, ...
-
Product Synthesis of APIs
APIs: Pitavastatin Calcium Hydrate, Rosuvastatin Calcium, Donepezil, Clopidogrel Bisulfate, Sarpogrelate Hydrochloride, Erdosteine, Pelubiprofen, (S)-Amlodipine Besylate 2.5 Hydrate, (S)-Pantoprazole Sodium Trihydrate
Intermediates of Statins:
MRV-1, ...
-
Product Amlodipine Bs., Dabigatran Etexilate Mesylate, Enalapril Maleate, Ezetimibe, Lercanidipine, Nebivolol, Rivaroxaban, Trimetazidine, Fluconazole, Itraconazole, Ketoconazole, Voriconazole, Bilastine, Cetirizine, Desloratadine, Levocetirizine
Ultratech India Limited manufactures a wide range of API's. Please refer our complete product list at www.ultratechindia.com.
Ultratech India Limited is a 30 year old transnational company specializing in the manufacturing of Active Pharmaceutical Ingredients (APIs) and ...
-
Product Paclitaxel
Paclitaxel is a naturally occurring antineoplastic agent and stabilizes tubulin polymerization. Paclitaxel can cause both mitotic arrest and apoptotic cell death. Paclitaxel also induces autophagy.
-
Product Nicotine USP/EP
Nico Orgo Marketing Pvt. Ltd. is a trusted Nicotine USP supplier in USA as well as one of the top Nicotine USP manufacturers in India. As a leading manufacturer of Nicotine USP/EP, we understand the importance of this compound and its broad range of applications.
Nicotine USP/EP is a high-grade ...
-
Product Pharmaceutical Grade Acetates
Our portfolio of Niacet acetates is backed by over 60 years of proven quality and delivery experience. Supported by an expert team and global supply network, we can meet your regulatory, technical and logistical needs to optimize your product formulation timelines. The portfolio consists of a range of acet...
-
Product API
Alchimica offers a wide range of active pharmaceutical ingredients for human and veterinary use. More information can be found in our Pharmaceuticals brochure.
-
Product NEW CRYSTAL FORM OF RESMETIROM
√Better chemical and mechanical stability than form I in
Rezdiffra
√Bioequivalence consistency with Rezdiffra
√Lower hygroscopicity than form I in Rezdiffra, et al.
-
Product ZLD, Zero Liquid Discharge
A Zero Liquid Discharge (ZLD) system is an advanced wastewater treatment process designed to eliminate liquid waste from a facility. By treating and recycling wastewater, ZLD systems recover water for reuse and concentrate the remaining waste into solid or liquid residues.
-
Product Lipids APIs and Intermediates
KD Pharma is the largest producer of omega-3 based APIs. Its unique technology mix produces Omega-3 drug substances of the highest quality and purity. We offer concentrates with EPA up to 99% purity, DHA up to 95% purity, and can custom manufacture concentrates of almost any other fatty acid.EPA fatty acid...
-
Product Choline Alfoscerate/L-Alpha-Glycerylphosphorylcholine
L-ycerylphosphorylcholine Powder-Mono-Diglyceride Fatty Acid Esters≥70%; L-alpha-Glycerylphosphorylcholine solution≥85%; Polyene Phosphatidylcholine≥75%; 1,2-Di(1-Z- 5,8,11,14,17 -eicosapentaenoyl)-sn-Glycero-3-Phosphatidylcholine≥75%;
1,2-Di(Z-4,7,10,13,16,19-docosahexaenoyl)-sn-Glycero-3-P...
-
Product Mannitol
Qingdao Bright Moon Seaweed Group Co,Ltd provides wide range of raw materials including Mannitol. We are the earliest domestic manufacturer of mannitol in China, including industry, food, pharmaceutical excipient and API. Contact us for more information.
-
Product Cyanocobalamin
Cyanocobalamin is a synthetic compound of vitamin B12 used to prevent and treat low blood levels.
-
Product Chlorhexidine series
Chlorhexidine Acetate, Chlorhexidine Hydrochloride and Chlorhexidine Gluconate Solution
-
Product Dexmedetomidine Hydrochloride
Product Name: Dexmedetomidine Hydrochloride
Molecular formula: C13H16N2·HCl
Molecular weight: 236.74
CAS No.: 145108-58-3
Shandong Chenlong Pharmaceutical Co Ltd offers a wide range of products which includes Dexmedetomidine Hydrochloride. Appearance: white or almost...
-
Product Ferrous Bisglycinate
Dr. Paul Lohmann® offers Ferrous Bisglycinate with API documentation. Through protection of the iron core by two glycine molecules, gastrointestinal side effects are prevented which makes Ferrous Bisglycinate an ideal API for treatment of iron deficiency anemia and hyperphosphatemia, especially for pre... -
Product Ursodeoxycholic Acid CAS 128-13-2
Chemical & Physical Properties:
Appearance: White crystalline powder
Assay :≥98.00%
Density: 1.128 g/cm3
Boiling Point: 547.1℃ at 760mmHg
Melting Point: 203-206℃
Flash Point: 298.8℃
Stability: Stable under normal temperatures ...
-
Product Follistatin API
- Regulates muscle growth
- Intended to increase lifespan and longevity by promoting muscle gain and enhancing overall physical vitality
- Manufactured in compliance with EU GMP standards
-
Product Prilocaine
Prilocaine is an amide local anaesthetic, with anaesthetic intensity and speed similar to that of lidocaine, but with a longer duration and weaker vasodilatory effect. Toxicity is lower than lidocaine. It is used clinically for local anaesthesia, especially for patients who contraindicate the use of epinep...
-
Product Berberine
Berberine is a bioactive compound extracted from the roots of the Berberis aristata plant. With a standardized concentration of upto 97%. Known for its potential health benefit in Blood Sugar Management & Weight Management.

-
Product Best-Selling Export Supplier Competitive Price Polyethylene Glycol/Peg 4000
Pharmaceutical Grade Polyethylene Glycol 4000 With High QualityMain use: pharmaceutical excipient, ointment bulk material and lubricant,tablet, capsules and ect. Due to the plasticity of PEG during the production process and its ability to improve the release of drugs from the tablet, high ...
-
Product RIFAMYCIN SODIUM
Rifamycin Sodium: Fine or granular red powder, an active ingredient used to treat tuberculosis, travelers' diarrhea.
-
Product Diosmin
Diosmin is a semisynthetic phlebotropic drug,a member of the flavonoid family.It is used with Hesperidin to control internal symptoms of hemorrhoids(piles).It is an oral phlebotropic drug used in the treatment of venous disease,ie,chronic venous insufficiency(CVI)and hemorrhoidal disease(HD),in...
-
Product Komcyst -contains Gynaecological patented ingredient
Komcyst is an innovative herb product extracted from fenugreek seeds and do not contain chemicals.
It is natural and promising dietary supplement effective for management of Polycystic Ovary Syndrome (PCOS).
Komcyst helps to:
Ø Increases insulin sensitizing activity &...
-
Product Nicotinamide Mononucleotide
Jinan Jianfeng Chemical Co., Ltd. Is An Enterprise Integrating R&D, Production And Sales. It Cooperates With Many Scientific Research Centers And Universities, Multiple Warehouses And R&d Factories. Focus On New Biological Drugs And New Resources That Promote Human Health.The Products Include Biolo...
-
Product Methocarbamol
CAS NO.:532-03-6Molecular Formula: C11H15NO5
Molecular Weight: 241.24
Usage:Skeletal Muscle Relaxant
Certification Note
1. Chinese registration number: Y20190008462
2. DMF Korean registration number: 20080110-81-D-54-02
3. DMF Taiwan registration number: 000306

-
Product Piperacillin Sodium, Tazobactam Sodium
Piperacillin Sodium, Tazobactam Sodium; API and FDF (8:1, strength 2.25g-3.375g-4.5g)
Xiangbei Welman Pharmaceutical Co., Ltd. manufactures and offers a wide-range of high-quality APIs and FDFs, including:
• Penicillins & Cephalosporins - Sterile Powder for...
-
Product Hyaluronans for medical devices and dermatological applications
Reshape the approach to Hyaluronans for medical devices and dermatological applications, by patented Hyaluromimethic technologyBMG Pharma’s. HYALUROMIMETHIC technology, represents a patented innovative approach based on biocompatible and bioreversible modifications of sodium hyaluronate at the level of its...
-
Product Sorafenib tosylate
Sorafenib tosylate (CAS No.: 475207-59-1) Indications: For the treatment of patients with advanced renal cell carcinoma (RCC). For the treatment of patients with unresectable hepatocellular carcinoma (HCC). For the treatment of patients with locally recurrent or metastatic, progressive, differentia...
-
Product Biotin CEP grade
ASMF: Available CEP: Available US DMF: Available GMP Certificate: Biotin CEP grade
-
Product SOMAI API Solutions
THC Extract >65%, ≤ 2% CBDTHC Purified Extract >75%, ≤ 3% CBD
CBD Extract >65%, ≤ 4% THC
CBD Broad Spectrum >75%, ≤ 0.3% THC
CBD Purified Extract >75%, ≤ 4% THC
CBD Isolate 98.0% – 102.0%
-
Product Mucic Acid (Galactaric Acid)
Color : White / Light Yellow
Form : Crystalline Powder
Loss on Drying (%) : Not more than 1%
Melting Point : Not less than 210°C
Solubility : Soluble in NaOH 1N
Insoluble in ethanol 96%
Absorbance of Solution : Not more than 0.040
Total Ash : Not more than 0.1% ...
-
Product SANAL® P+ Pharmaceutical Sodium Chloride (API)
SANAL® P+ Sodium Chloride Pharmaceutical Quality is for specific market requirements (eg. the Chinese and Russian markets) as active ingredient in both parenteral and peritoneal solutions and a base material for hemodialysis and hemofiltration solutions as well as other pharmaceutical applic...
-
Product Uni-Carbomer 981G / Carbomer
Uni-Carbomer 981G polymer can be used to develop clear, low-viscosity lotions and gels with good clarity. Additionally, it can provide emulsion stabilization of lotions and is effective in moderately ionic systems. The polymer has long flow rheology similar to honey.
NM-Carbomer 981G ...
-
Product Methotrexate Sodium GMP Manufacturer Cas No.7413-34-5
Methotrexate Sodium GMP Manufacturer Cas No.7413-34-5
N+1 in the base of Methotrexate API.
Quantity per batch about 30KG.
Monthly production capability 500KG.
Delivery time: stable stock, prompt delivery.
We supply series of Methotrexate products...
-
Product Retatrutide
Retatrutide is a triple agonist peptide of glucagon receptor (GCGR), glucose-dependent insulin-stimulating polypeptide receptor (GIPR), and glucagon-like peptide-1 receptor (GLP-1R). Retatrutide treats obesity in adults with significant weight loss.
Products are for scientific use only !
-
Product α-Ketoacid Calcium APIs & Tablets
α-Ketoacid Calcium APIs including: α-Ketoleucine Calcium (CAS NO. 51828-95-6), α-Ketovaline Calcium (CAS NO. 51828-94-5), α-Ketophenylalanine Calcium (CAS NO. 51828-93-4), D,L-α-Ketoisoleucine Calcium (CAS NO. 66872-75-1) and D,L-α-Hydroxymethionine Calcium (CAS NO. 4857-44-7)
Compound α...
-
Product Paclitaxel
Antitumor drugs. It is used to treat metastatic breast cancer and metastatic ovarian cancer. A broad-spectrum anti-tumor plant drug used to treat ovarian cancer, breast cancer and other diseases
-
Product D-Glucosamine Hydrochloride
This product is a kind of natural chitin extraction. mainly applied in medical treatment drugs. It has important physiological functions to human body, It can promote the body's synthesis of mucopolysaccharide and is conducive to the articular cartilage's metabolism and repair, with significant anti-inflam...
-
Product Nicorandil
In July 2023, nicorandil verifiy and certify by the CFDA API quality and can be used in the production of preparation, and nicorandil is used for the treatment of angina. The percent of nicorandil which in our product exceeds 99.0%.
-
Product Norepinephrine Bitartrate
Norepinephrine is a sympathomimetic used in the control of blood pressure during various hypotensive states and as an adjunct treatment during cardiac arrest.
-
Product Trypsin
BGB can supply Trypsin of EP/BP/USP standard with customizable specifications, from either porcine or bovine source.

-
Product Hyaluronic Acid/Hyaluronan/Sodium Hyaluronate
Hyaluronic Acid, is a biological polymer that is naturally present in the human body. It’s been used for decades in medical devices in an array of therapeutic fields including ophthalmology, rheumatology, aesthetic medicine. New fields for this highly effective API are continuin...
-
Product Hydrochlorothiazide
Hydrochlorothiazide
Therapeutic Area: Cardiovascular system • EU DMF available • US DMF no. 17599 available • Japanese DMF available • CEP available
DISCLAIMER
Products protected by valid patents are not offered for sale in countries where the sale of ...
-
Product Human API
Anti-Infective,Cardiovascular & Cerebrovascular, Biological Medicine, Antipyretics & Analgesics & Antiphlogistics, Contrast Medium,Gastrointestinal Agents,Antidiabetics,etc.
-
Product Avibactam Sodium
Zhejiang Yongning Pharmaceutical Co. Ltd offers a wide range of products which includes Avibactam Sodium. It belongs to APIs category. Storage: cool and dry , protected from light. Contact us for more information.

-
Product Ethanol 96
Kimia offers a wide range of products which includes ethanol 96. Uses and applications: it is used in the manufacture of pharmaceutical products. Ethanol 96 can be supplied as either synthetic or fermentation ethanol. Contact us for more information.
-
Product Pharma Freight
Life Couriers are the global experts in GDP-compliant transportation for the pharma industry.
With the highest focus on integrity and process quality, we fully comply with the ever-changing regulatory requirements across national borders, providing safe and secure cold chain solutions across the...
-
Product Minoxidil USp
• Chemical Name: Minoxidil
• Molecular Formula: C9H13N5O
• CAS Number: 38304-91-5
• Appearance: Typically a white to off-white crystalline powder.
• Solubility:
• Soluble in water and alcohol. • Practically insoluble in ether. • Identifica...
-
Product Doxycycline Monohydrate
Yangzhou liberty pharamceutical co. Ltd offers a wide range of product which includes doxycycline monohydrate. Contact us for more information.
-
Product (+)-Cloprostenol isopropyl ester
Taizhou Crene Biotechnology Co.,Ltd. manufacture of Bimatoprost,Latanoprost,Travoprost,Tafluprost,Lubiprostone ,Cloprostenol sodium,Alprostadil,Tafluprost ethyl amide,Bimatoprost isopropyl este.
-
Product API
Azithromycin, Clarithromycin, Roxithromycin, Erythromycin, Potassium Sodium, Dehydroandroan, Clindamycin Hydrochloride, Lincomycin Hydrochloride, Erythromycin Ethylsuccinate, Erytromycin, Acetylspiramycin, Sulfamethoxazole, Sulfadiazine, Noromycin sulf...
-
Product Wastewater and sludge disposal service
We treat up to 400,000 m3 per year of liquid waste, thanks to our treatment centre that combines different technologies, from conventional biological treatments to advanced treatments such as wet oxidation. Mindful of the significance not only of waste treatment but also of the recovery of valuable sub...
-
Product Nucleotides, Nucleotide triphosphates (NTPs) & Deoxynucleotide triphosphates (dNTPs)
We develop a broad range of Nucleotides, Nucleotide triphosphates (NTPs) and Deoxynucleotide triphosphates (dNTPs).
Nucleotide diphosphates:
Uridine 5'-diphosphate disodium salt (UDP);
Guanosine 5'-diphosphate disodium salt (GDP);
Cytidine 5'-diphosphate disodium salt (CDP...
-
Product Vitamin B12 Series Products
Our Vitamin B12 series products: Cyanocobalamin, Mecobalamin, Hydroxocobalamin, Cobamamide, food additive and feed additive.

-
Product Haloperidol Decanoate Injection
A slightly amber
coloured oily solution filled in glass container.
-
Product Icodextrin
Icodextrin peritoneal dialysate is currently the world’s most advanced peritoneal dialysate with multiple clinical advantages. Icodextrin is the key API in it.
-
Product Landing EU Pharma
Marketing authorization is granted only to an EU-based applicant. Medicines imported into the EU must be QC tested and QP batch-released before entering the EU. Our QP and EU batch release services can assist you with EU imports of sterile and non-sterile medicinal products, as well as ATMP and I...
-
Product Sacubitril Intermediate(to produce LCZ 696,Sacubitril/Valsartan)
Sinobright Pharma initiated and invested the project of LCZ 696 key intermediates and received technical patents related to synthetic routes. Comparing to other manufacturers, we have significant advantages in production technology and cost.
-
Product Sevoflurane API, Propofol API and Glycofurol excipient
High quality APIs, Minimum impurities offered by Troikaa Pharmachem Pvt. Ltd.; a wholly owned subsidiary of Troikaa Pharmeceuticals Ltd. • Sevoflurane • Propofol • Remifentanyl • Ketamin • Dexmedetomidine • Midazolam • Rocuronium Bromide • Dequalinium • Glycofurol (Excipient)
-
Product Norepinephrine tartrate (CEP / US-DMF)
Transo-Pharm Handels-GmbH offers a wide range of API products which includes Norepinephrine tartrate (CEP / US-DMF). It belongs to API category. Contact us for more information. Please check for all products our product list.
-
Product KETOPROFEN
Ketoprofen is used for pain relief. It relieves pain and inflammation in conditions like rheumatoid arthritis, ankylosing spondylitis, osteoarthritis, gout, muscle pain, low back pain and toothache.
-
Product Trypsin
Trypsin is an enzyme that aids with digestion. An enzyme is a protein that speeds up a certain biochemical reaction. Trypsin is found in the small intestine. It can also be made from fungus, plants, and bacteria.
-
Product SULFADIAZINE
Chongqing Kangle Pharmaceutical Co. Ltd. provides wide range of pharmaceutical products which includes piperaquine phosphate. It belongs to active pharmaceutical ingredients category. Properties: white to light yellow crystalline powder. Use : it is antimalarial. Contact us for more information.
-
Product CUSTOMS SYNTHESIS OF PEPTIDES
BCN Peptides is an industrial manufacturer with the capacity to produce from milligram to multi-kg batches of pharmaceutical peptides.
We are experts in the synthesis of long and complex New Chemical Entities.BCN Peptides ensures the highest qualities and shortest times thanks to our experience ...
-
Product API and Intermediates
Active Pharmaceutical Ingredients
-- Artemether
-- Lumefentrine
-- Deferasirox
-- Ofloxacin
-- Oxymetazoline
-- Xylometazoline
-- Acyclovir
-- Valgancyclovir
-- Methylcobalamine
-- Tamsulosin
-
Product Fine Chemicals
MP Biomedicals offers a diverse selection of high-quality chemicals to support a variety of bulk requests and manufacturing needs.
Keep your projects moving with all the right compounds, including: • Antibiotics • Immunology & Cell Biology • Nucleotides ...
-
Product Progesterone
Progesterone is widely used in oral contraceptives (preventing preterm birth, infertility) and hormone replacement therapy (HRT).With different applications, it can also help for preventing premenstrual syndrome (PMS), absence of menstrual periods (amenorrhea), reducing abnormal thickening of the lining of...
-
Product Sodium hyaluronate
HA is a natural moisturizing agent, widely used in medical fields.sodium hyaluronate has good functions ofmoisturizing, lubricating, viscoelasticity, repairing damage cartilage, inhibition of inflammation, painrelief, etc. And it can be used in ophthalmic viscosurgical devices and intra-articular injection...
-
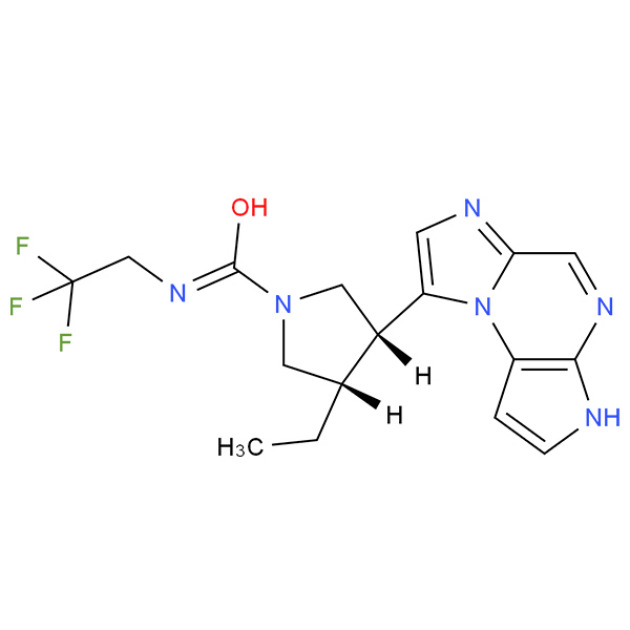
Product SUZETRIGINE
Suzetrigine(VX-548) is an orally active and specific NaV1.8 inhibitor. Suzetrigine has analgesic activity and can be used to study acute pain and neurotransmission.As compared with placebo, VX-548 at the highest dose (a 100-mg oral loading dose of VX-548, followed by a 50-mg maintenance dose every 12 hours...
-
Product Cyanocobalamine (vitamin B12) and its derivatives
Hepartex distributes a wide range of APIs which includes Cyanocobalamine (vitamin B12) and its derivatives. Please contact us for more information.
-
Product Sodium lactate solution
Sodium lactate solution is mainly used to regulate the balance of electrolytes in the human body and it also works to treat dehydration caused by diarrhea and poisoning caused by diabetes and gastritis. Sodium lactate is used for oral drug as well as injectable one. Clinically, sodium lactate solution is a...
-
Product Valsartan
Resolution Limited offers a wide range of products which includes Valsartan. Contact us for more information.
-
Product Calcium gluconate
This product is available in two different grades, for oral and injection.
Calcium gluconate, as a medication, can supplement calcium and prevent/treat calcium deficiency disorders such as osteoporosis, tetany, skeletal dysplasia, and rickets.

-
Product Thio-Tepa
Tiefenbacher api & ingredients gmbh & co offers a wide range of products which includes thio-tepa. Contact us for more information.
-
Product Finerenone
Product name: Finerenone
Alias: Finerenone (BAY94-8862); (4S)-4-(4-cyano-2-methoxyphenyl)-5-ethoxy-2,8-dimethyl-1,4-dihydro-1,6-naphthyridine-3-carboxamide; (4S)-4-(4-cyano-2-methoxyphenyl)-5-ethoxy-2,8-dimethyl-1,4-dihydro-1,6-naphthyridine-3-carboxamide; BAY94-8862; BAY948862; 1,6-Naphthyridine-3-...
-
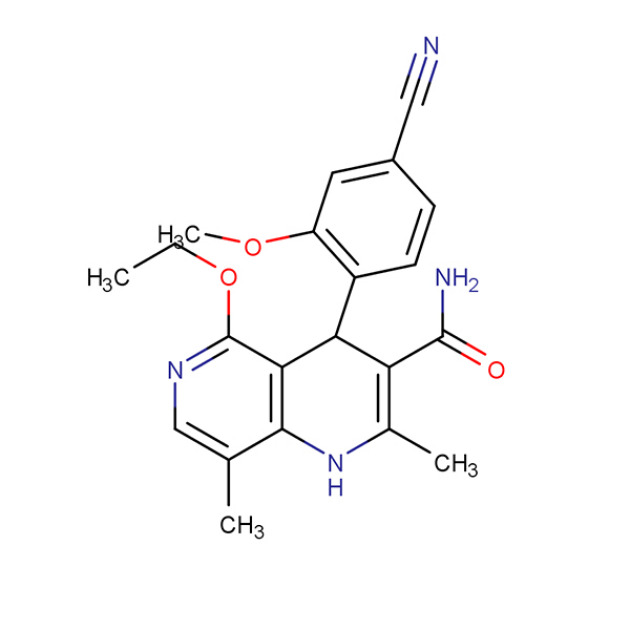
Product Resmetirom
product name: Resmetirom
Alias: MGL-3196; 920509-32-6; VIA-3196; 2-(3,5-dichloro-4-((5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yl)oxy)phenyl)-3,5- dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile; MGL 3196; Resmetirom [USAN]; MGL3196; RE0V0T1ES0
CAS No. 920509-32-6
...
-
Product Upadacitinib
Product name: Upadacitinib
English Synonyms: Upadacitinib; ABT-494; ABT-494(Upadacitinib) free base; Upadacitinib (ABT-494); ABT-494 (Upadacitinib); Upadacitinib (Rinvoq); ABT-494. ABT494; ABT 494; CS-2730
CAS No.: 1310726-60-3
Molecular Formula: C17H19F3N6O
...
-
Product Apalutamide
Product name: Apalutamide
Alias: ARN-509;4-(7-(6-(6-cyano-5-(trifluoroMethyl)pyridin-3-yl)-8-oxo-6-thioxo-5,7-diazaspirooctan-5-yl)-2-fluoro-N-MethylbenzaMide; AR509;Benzamide,4-[7-[6-[6-cyano-5-(trifluoromethyl)-3-pyridinyl]-8-oxo-6-thioxo-5,7-diazaspirooctan-5-yl AR509;Benzamide,4-[7-[6-cy...
-
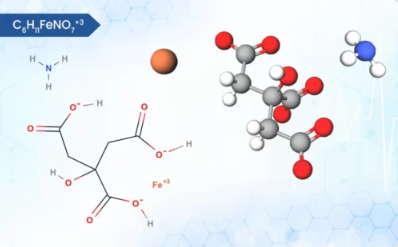
Product Calcium Pidolate
aCalcium Pidolate has been reported to be better absorbed than other calcium salts, to lower the levels of parathyroid hormone (PTH) and to raise those of growth hormone (GH). It might have value in the treatment of involutional osteoporosis. There is a correlation between cumulative calcium dose at the en...
-
Product Liposomal Calcium
Liposomal calcium is the encapsulation of calcium ions within a liposome structure for the potential delivery of calcium ions across the cell membrane or enhanced absorption.
-
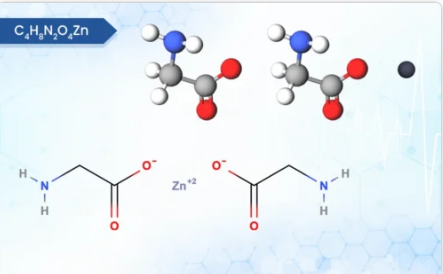
Product ZINC BISGLYCINATE
Zinc Bisglycinate is a unique and well-tolerated form of zinc, a mineral essential for numerous bodily functions. Physically, it appears as a white, odourless powder with a slightly sweet taste. Unlike some other zinc supplements, it boasts low hygroscopicity, meaning it resists absorbing moisture from the...
-
Product CALCIUM PROPIONATE
Calcium propionate is a chemical compound that is used in animal nutrition, primarily in the feed industry. It serves as a preservative and source of calcium.
-
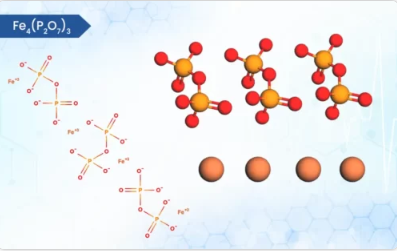
Product Ferric Pyrophosphate
Ferric pyrophosphate, a key iron source in some dietary supplements, boasts distinct physical and chemical properties. It exists as a yellowish-white to beige crystalline solid [1]. Unlike many iron compounds, it’s practically insoluble in water, rendering it odorless and tasteless [1]. Its white colour is...
-
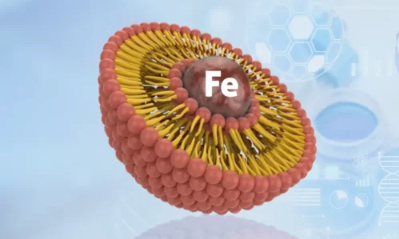
Product Liposomal Iron
Liposomal iron isn’t a single, well-defined compound but rather a delivery system for iron. It encapsulates iron molecules within microscopic spheres made of phospholipids, the same fatty molecules that form cell membranes. These spheres, called liposomes, are typically colorless to slightly yellow and ran...
-
Product Iron Sucrose
Iron sucrose is a complex molecule used to replenish iron stores in individuals with deficiency, particularly those with chronic kidney disease. Unlike oral iron supplements, iron sucrose is administered intravenously. Physically, it appears as a brown to reddish-brown sterile solution and is odorless. Int...
-
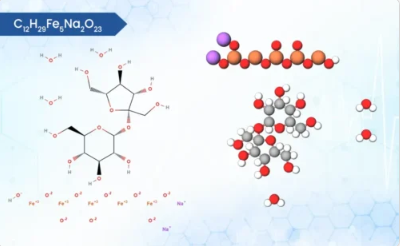
Product FERRIC AMMONIUM CITRATE
Ferric ammonium citrate (FAC), also known as ferric ammonium citrate scales or ferric ammonium citrate trihydrate exhibits interesting physical and chemical properties. It is a water-soluble coordination complex with the chemical formula FeC6H5O7(NH4)

-
Product FERRIC MALTOL
Ferric maltol is a chemical compound used as an iron supplement. Ferric maltol, formally named (3-hydroxy-2-methyl-4-pyronato)iron(III), is a coordination complex containing iron(III) centrally chelated by a maltol (3-hydroxy-2-methyl-4-pyrone) ligand. This complexation enhances the solubility and bioavail...
-
Product FERRIC CARBOXYMALTOSE
Ferric carboxymaltose is a unique iron replacement therapy medication with interesting scientific and chemical properties. Unlike traditional iron supplements, it’s a carbohydrate-based complex formulated with iron (III) ions. This complex gives ferric carboxymaltose a distinct lack of color and odor, maki...
-
Product FERRIC DERISOMALTOSE
Ferric Derisomaltose also known as Ferric Isomaltoside, is one of the latest injectable iron accepted worldwide due to its much lesser hypersensitivity reactions & anaphylactic shock.
-
Product Liposomal Iron, Liposomal Calcium, Liposomal Magnesium, Liposomal Zinc, Liposomal Vitamin C, Liposomal ALA, Liposomal Glutathione, Liposomal Curcumin, Liposomal Vitamin B12, Liposomal Vitamin K2, Liposomal Thiamin
Liposomal Iron - It encapsulates iron molecules within microscopic spheres made of phospholipids, the same fatty molecules that form cell membranes. Liposomal Calcium - It is the encapsulation of calcium ions within a liposome structure for the potential delivery of calcium ions across the cell m...
-
Product Liposomal Iron, Ferrous Bisglycinate, Ferric Citrate, Iron Polymaltose, Sucroferric Oxyhydroxide, Iron Isomaltoside, Ferric Derisomaltose,Ferric Pyrophosphate, Ferric Carboxymaltose,Ferric Ammonium Citrate,Ferrous Ascorbate,Ferric Maltol.
WBCIL is a leading manufacturer of Iron supplementations which are in the form of chelated iron,Iron complex, Liposomal Iron & Injetable iron. The products qualities are confirmed by different characteristics analysis like FTIR,NMR,XRD,Mass spectroscopy & many more. Many o...
-
Product L-Methylfolate ,Boron Citrate, Boron Glycinate, Sucroferric Oxyhydroxide, Enclomiphene Citrate , Sodium Butyrate Food / USP, Calcium Butyrate Food Grade , Ulipristal Acetate, Ascorbyl Palmitate, Dimethyl Fumarate, L Ornithine L Aspartate,
L-Methylfolate Boron Citrate
Boron Glycinate
Sucroferric Oxyhydroxide
Enclomiphene Citrate
Sodium Butyrate Food / USP
Calcium Butyrate Food Grade
Ulipristal Acetate
Ascorbyl Palmitate
Dimethyl Fumarate
L Ornithine L Aspartate
-
Product Calcium Lactate Gluconate,Calcium Levulinate,Calcium Pidolate,Calcium Butyrate,Coral Calcium, Calcium Gluconate,Calcium Aspartate,Calcium Orotate,Calcium Bisglycinate,Calcium Acetate, Liposomal Calcium,Calcium Citrate,Calcium Citrate Malate, Milk Calcium
Calcium is essential for bone health, muscle function, and cellular activity. West Bengal Chemical Industries Limited (WBCIL) offers an extensive range of high-quality calcium compounds to meet diverse industry needs. Our portfolio includes Calcium Gluconate for deficiency treatment, Calcium Lactate G...
-
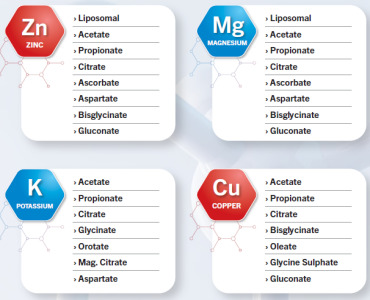
Product ZINC Acetate,ZINC Citrate,ZINC Bisglycinate,ZINC Gluconate,MAGNESIUM Acetate,MAGNESIUM Citrate,MAGNESIUM Glycinate,POTASSIUM Acetate,POTASSIUM Propionate,POTASSIUM Glycinate,POTASSIUM Orotate,COPPER Acetate,COPPER Citrate, COPPER Glycinate
ZINC - Liposomal, Acetate, Propionate, Citrate, Ascorbate, Aspartate, Bisglycinate, GluconateMAGNESIUM- Liposomal, Acetate, Propionate, Citrate,Ascorbate, Aspartate, Bisglycinate, Gluconate
POTASSIUM - Acetate, Propionate , Citrate› Glycinate , Orotate, Mag. Citrate, Aspartate qq...
-
Product API portfolio
Siegfried offers a broad portfolio of active pharmaceutical ingredients including controlled substances, focused on anesthetics, analgesics, decongestants, addiction treatment, and a variety of other therapeutic indications. In addition, we offer pharma grade caffeine for use in pharmaceutical and nutritio...
-
Product Sterile Dosage Filling and Lyophilization
IDT Biologika GmbH provides a wide range of pharmaceutical services which includes sterile dosage filling in vials and pre-filled syringes. With a facility size of 3,600m2, this integrated pharmaceutical/ biological site houses more than 7 filling lines for the aseptic filling of syringes, vials, ampoules ...
-
Product Drug Substance Manufacturing for Viral Vaccines, Gene & Immune Therapeutics, Oncolytic Viruses and Viral Vectors
Drug Substance Manufacturing for pre-clinical, clinical and commercial supply- Validation of aseptic processes - Cell and virus banking - Using own seed cell banks: VERO and DF-1, Duck cell line (AGE-1) - API production using different TechnologiesMVA Know-How IDT Biologika is considered the global lea...
-
Product IDT Biologika as a Contract Development and Manufacturing Organization
IDT Biologika is an international leader in the contract development and manufacturing of biologics. The company focuses on the customized development and manufacturing of viral vaccines (phases I, II, III), gene therapeutics, immunotherapeutics, oncolytic viruses as well as sterile liquid and lyophilized ...
-
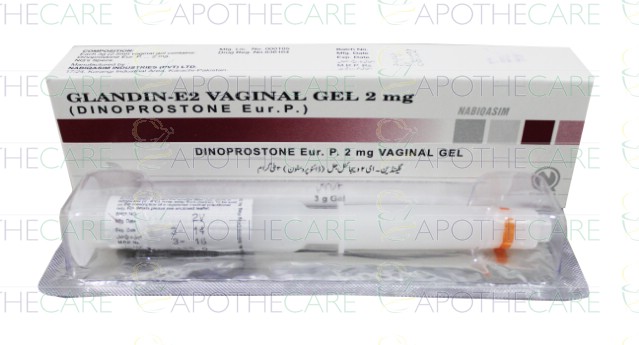
Product Prostaglandin e2 (Pessaries) / Dinoprostone E2 / Glandin E2 (Tablet & Gel)
1- Glandin E-2 3Mg Vaginal Tablets 1S (Pack Size 1 X 1S)2- Glandin E-2 2Mg Vaginal Gel Generics: Dinoprostone Used For: Labour Inducer How it works: Dinoprostone is a prostaglandin of the E series with actions on smooth muscle; the endogenous substance is termed prostaglandin E2. It induces co...
-
Product Taste Masked Coated Granules of Clarithromycin & Azithromycin
White to off white tasteless free flowing granules Recommended for Dry Syrup / Suspension
-
Product Tripotassium Citrate Monohydrate (TPC)
Jungbunzlauer is the worldwide leading supplier of Tripotassium Citrate Monohydrate (TPC) as an API with GMP quality and documentation, including Type II US DMF and CEP. TPC serves as potassium source in a wide range of nutritional and pharmaceutical applications. As an API, TPC is mainly used as...
-
Product Trimagnesium Citrate Anhydrous (TMC)
GMP certificates and a CEP can be provided for Trimagnesium Citrate Anhydrous (TMC). Jungbunzlauer is prepared to expand its regulatory documents according to the customer needs. To facilitate your licensing activities, Jungbunzlauer strives for high-quality regulatory support and provides four API...
-
Product Trisodium Citrate Dihydrate (TSC)
Jungbunzlauer provides Trisodium Citrate Dihydrate (TSC) with a GMP certificate, Type II US DMF and a CEP for drug product registrations. TSC is mostly used in its dihydrate form, has excellent pH regulation and buffering properties. As an API, TSC is widely used as anticoagulant, in rehydration so...
-
Product Active Pharmaceutical Ingredients (APIs)
• Citric Acid Anhydrous • Tripotassium Citrate Monohydrate • Trimagnesium Citrate Anhydrous • Trisodium Citrate Dihydrate

-
Product Custom and Flow Chemistry
Our continuous flow technology platform enables access to better synthetic strategies which are shorter, offer assured safety, reduce environmental impact and control of by-products. In addition, improved yields and improved reaction selectivity is achievable. With the reaction under tight control, the...
-
Product Dolutegravir Sodium, Oseltamivir Phosphate, Valacyclovir, Valganciclovir, Budesonide, Fenoterol HBr, Fluticasone Propionate, Fluticasone Furoate, Formoterol Fumarate, Ipratropium Bromide, Montelukast, Salbutamol, Salmeterol Xinafoate, Tiotropium Bromide
Ultratech India Limited is a 30 year old transnational company specializing in the manufacturing of Active Pharmaceutical Ingredients (APIs) and a diverse range of branded generic Finished Dosage Forms (FDFs) at its cGMP certified state-of-the-art manufacturing facilities in India, with a global presen...
-
Product Duloxetine, Vigabatrin, Vortioxetine, Zonisamide, Avanafil, Dapoxetine, Sildenafil Citrate, Tadalafil, Vardenafil, Glibenclamide, Linagliptin, Lansoprazole API/Pellet, Omeprazole API/Pellet, Vonoprazan Fumarate
Ultratech India Limited is a 30 year old transnational company specializing in the manufacturing of Active Pharmaceutical Ingredients (APIs) and a diverse range of branded generic Finished Dosage Forms (FDFs) at its cGMP certified state-of-the-art manufacturing facilities in India, with a global presen...
-
Product Solifenacin Succinate, Ibandronate Sodium, Sodium Alendronate, Zoledronic Acid, Brinzolamide, Dorzolamide HCl, DOTA (Tetraxetan), Gadobutrol and Gadoterate Meglumine.
Ultratech India Limited is a 30 year old transnational company specializing in the manufacturing of Active Pharmaceutical Ingredients (APIs) and a diverse range of branded generic Finished Dosage Forms (FDFs) at its cGMP certified state-of-the-art manufacturing facilities in India, with a global presen...
-
Product Vonoprazan Fumarate
Ultratech India Limited manufactures a wide range of API's. Please refer our complete product list at www.ultratechindia.com.
Amlodipine Besilate, Dabigatran Etexilate Mesylate, Enalapril Maleate, Ezetimibe, Lercanidipine HCl, Nebivolol HCl, Rivaroxaban, Rosuvastatin Calcium, Trimetazidine H...
-
Product Nicotine Polacrilex USP
Nicotine Polacrilex USP, also known as nicotine gum, is a specialized form of nicotine that is widely used as a nicotine replacement therapy (NRT) product. It plays a crucial role in helping individuals reduce their dependence on tobacco by providing controlled amounts of nicotine. As a distinguished Nicot...
-
Product Solvent stripping unit
A tailor-made solvent stripping unit is an industrial system precisely customized to meet the specific requirements of a given process or product. Engineered to efficiently remove residual solvents from liquid mixtures, it is designed with a focus on minimizing operational costs.
-
Product Aerobic system
These secondary treatments are used to break down any biodegradable organic matter and to remove non-settleable solids that cannot be separated by physical treatment.
-
Product Chemical-physical plant
The chemical-physical treatment allows the processing of heavily polluted wastewater, including those with suspended metals, which cannot be treated using biological systems. These wastewater treatments utilize the chemical processes of flocculation and sedimentation, combined with the physical pr...
-
Product Calcium carbonate
MAGNESIA GmbH provides a wide range of products which includes calcium carbonate. Contact us for more information.
-
Product Magnesium hydroxide
MAGNESIA GmbH provides a wide range of mineral raw materials which includes Magnesium hydroxides. Contact us for more information.
-
Product BERBERINE HYDROCHLORIDE
It is from Natural Extract. It should be Yellow crystalline powder,inodorous,extreme bitter .Assay is mort than 97%
-
Product RUTIN
Sichuan province yuxin pharmaceutical co. Ltd offers a wide range of products .It is light yellow-green powder. no smell, no taste.
-
Product ROTUNDINE
It is White or slightly yellow crystal granule, Odorless, Tasteless.It turns yellow when exposed to light and heat. Soluble in chloroform, slightly soluble in ethanol or ether, insoluble in water; soluble in dilute sulfuric acid.The assay is 98.5-102%.
-
Product VITAMIN D3
Sichuan province yuxin pharmaceutical co. Ltd offers a wide range of products which includes l vitamin D3. It is obtained from the ultra-violet light irradiation and thermal isomerization of 7-dehydrocholesterol. The pharma grade is white crystal powder ,the oil form is yellow to light brown oil,resi...
-
Product Pantoprazole Sodium
Used for active peptic ulcer (gastric, duodenal ulcer), reflux esophagitis and Zollinger-Ellison syndrome.
-
Product Betahistine Hydrochloride
Mainly used for Meniere's syndrome, vascular headache and cerebral arteriosclerosis, and can be used to treat acute ischemic cerebrovascular diseases, such as cerebral thrombosis, cerebral embolism, transient cerebral insufficiency, etc.; it's also effective for hypertension-induced vertigo, tinnitus, etc....
-
Product Benzocaine
Benzocaine is a local anesthetic used for surface anesthesia, which has the effect of reducing pain, relieving pain, and relieving itching. It can be used for pain relief and relieving itching in wounds, ulcer surfaces, burns, skin abrasions, and hemorrhoids.
-
Product DAPOXETINE HYDROCHLORIDE
This product is indicated for premature ejaculation(PE) in men aged 18 to 64 years.
-
Product OSELTAMIVIR PHOSPHATE
For the treatment and prevention of influenza A and B in adults and children one year and older.
-
Product Heparin Sodium
Product Name: Heparin Sodium
Molecular formula: (C12H16NS2Na3)20
CAS No.:9041-08-1
Shandong Chenlong Pharmaceutical Co Ltd offers a wide range of products which includes Heparin Sodium. Appearance: White or almost white powder. Contact us for more information.
-
Product Enoxaparin Sodium
Product Name: Enoxaparin Sodium
Molecular formula: C57H82N4Na4O53S3
CAS No.: 679809-58-6
Shandong Chenlong Pharmaceutical Co Ltd offers a wide range of products which includes Enoxaparin Sodium. Appearance: White or almost white powder. Contact us for more information.
-
Product Alanyl-Glutamine
Product Name: Alanyl-Glutamine
Molecular formula: C8H15N3O4
Molecular weight: 217.22
CAS No.:39537-23-0
Shandong Chenlong Pharmaceutical Co Ltd offers a wide range of products which includes Alanyl-Glutamine. Appearance: White or almost white crystals or crystalline ...
-
Product Faropenem Sodium
Product Name: Faropenem Sodium
Molecular formula: C12H14NNaO5S/2H2O
Molecular weight: 352.34
CAS No.:122547-49-3
Shandong Chenlong Pharmaceutical Co Ltd offers a wide range of products which includes Faropenem Sodium. Appearance: White to pale yellow crystals or...
-
Product Mineral Gluconates
Dr. Paul Lohmann® offers various highly bioavailable Mineral Gluconates with API documentation - ASMFs and for Ferrous Gluconate a CEP - for the registration of a drug with the health authorities. Please contact us for more information.
-
Product Sulopenem CAS 120788-07-0
Antibacterial. Sulopenem (CP-70429) is an orally active, parenteral penem antibiotic with broad-spectrum activities against Gram-positive and Gram-negative bacteria. Sulopenem has the potential for urinary tract infections and intra-abdominal infections treatment. Sulopenem is inactive against Pseudomonas ...
-
Product Bempedoic Acid
Bempedoic Acid inhibits ATP citrate lyase, acts upstream of HMG-CoA reductase in the cholesterol synthesis pathway, lowers endogenous cholesterol and reduces elevated LDL-C levels by up-regulating the LDL receptor, mitigating muscle-related side effects. Unlike statins, which are currently the mainsta...
-
Product Methylphenidate Hydrotalcite
Methylphenidate hydrochloride is a central excitatory drug that directly excites the respiratory centre of the medulla oblongata and has a milder action. It is mainly used to treat minor disorders of brain function, attention deficit disorder (hyperactivity syndrome in children, etc.), and to eliminate bar...
-
Product Nifedipine
Nifedipine is a dihydropyridine calcium antagonist, which can inhibit the uptake of Ca2+ by myocardium and vascular smooth muscle, dilate coronary arteries, increase coronary artery blood flow, and improve myocardial tolerance to ischemia, and at the same time dilate the small peripheral arteries, reduce p...
-
Product Paliperidone
Paliperidone is an active metabolite of the atypical antipsychotic drug risperidone. Paliperidone is a derivative of benzisoxazole, the active metabolite of risperidone, 9-hydroxy risperidone, a new antipsychotic drug.
-
Product Best-Selling Export Supplier Competitive Price Polyethylene Glycol/Peg 6000
low price high quality pharmaceutial grade peg 6000Main use: pharmaceutical excipient, ointment bulk material and lubricant,tablet, capsules and ect. Due to the plasticity of PEG during the production process and its ability to improve the release of drugs from the tablet, high molecular we...
-
Product Microcrystalline Cellulose
Microcrystalline cellulose is a purified, partially depolymerized cellulose, a white, odorless, tasteless, crystalline powder consisting of porous particles.Main application:1. This product can be used as anticoagulant, emulsifier, dispersant, adhesive, tablet and tissue improver, etc. It can also be used ...
-
Product Polysorbate 80
Soluble in water, soluble in ethanol, vegetable oil, insoluble in mineral oil. Gelatinous at low temperature, after being heated to recover. Very smelly, slightly bitter taste. It is helpful to the dispersion of phthalocyanine green.Main application:
1.A wetting agent used for suspension. Nonionic surfa...
-
Product Sorbitol
Sorbitol is a sugar alcohol, which is a poorly digestible carbohydrate that either naturally occurs in fruits or is semi-artificially produced and added as a low calorie sweetener to various commercial foods.
Sorbitol is chemically similar to fructose and mannitol.Other names for sorbitol: ...
-
Product L- Rhamnose
Rhamnose is a naturally-occurring deoxy sugar.It can be classified either as a methyl-pentose or a 6-deoxy-hexose.Rhamnose occurs in nature in its L-form as L-rhamnose(6-deoxy-L-mannose).This is unusual since most of the naturally-occurring sugars are in D-form.Exceptions are the methyl pentos...
-
Product Troxerutin
Troxerutin is a derivative of the natural bioflavonoid Rutin extracted from Sophora japonica.Troxerutin is best suited for the treatment of the pre-varicose and varicose syndrome,varicose ulcers,trombophlebitis,post-phlebitic conditions,chronic venous deficiency,and he...
-
Product Rutin
Description
Rutin is a flavanoid obtained by extraction from the plant raw material,Sophora japonica L.It is vital in its ability to increase the strength of the capillaries and to regulate their permeability.It assists Vitamin C in keeping collagen in healthy condition...
-
Product Quercetin
Quercetin is a phytochemical that is part of the coloring found in the skins of sophora japonicas and red onions.It has been isolated and is sold as a dietary supplement.Quercetin is a powerful antioxidant.It is also a natural anti-histamine,and anti-inflammatory.Research shows that quercetin may h...
-
Product Apixaban intermediate
Our company produces Apixaban intermediates in various CAS numbers.Apixaban API:CAS 503612-47-3
Apixaban intermediate:
CAS 27143-07-3
CAS 473927-63-8
CAS 545445-44-1
CAS 1267610-26-3
CAS 503615-03-0
CAS 503614-91-3
CAS 536760-29-9
CAS 503615-03-0
CAS 536759-91-8
What are Active Pharmaceutical Ingredients (API)?
Drugs are composed of two major components: – Active pharmaceutical ingredient (API), and excipients. APIs vary from drug to drug and are responsible for creating the intended biological, physiological or therapeutic effect within the recipient's (patient's) body. A drug can contain one or more APIs depending on its purpose and the synergistic actions of the APIs.
Active pharmaceutical ingredients (APIs) are the therapeutically active substances or chemical components of drug products (capsules, tablets, syrups, etc.) that produce an intended pharmacological effect.
The APIs within drugs are conveyed into the body through inactive components known as excipients, which usually make up the bulk of the drug. They facilitate the ingestion, absorption, and distribution of the active ingredients. These excipients serve as vehicles and are usually inert or biologically inactive.
Source APIs from our marketplace of 900+ global API manufacturers and 2,500+ API solutions in categories such as Amino Acids, Enzymes, Antibiotics, Antibodies, Sera and Vaccines and more.
What is the purpose of APIs?
APIs are the primary ingredients contained in drugs and medicines. They play a significant role in drug production and produce pharmacological effects within the body. The pharmacological effects produced, directly or indirectly, influence the diagnosis, symptom relief, mitigation, treatment, cure, or prevention of diseases. APIs also modify, correct, or enhance the physiological function and structure of chemicals, cells, tissues, and organs within the body. In simple terms, they make the drug or medicine work.
What is the overall size of the API market?
With the exponential rise in the global population, the growing demand for a therapeutic solution to critical and chronic diseases, change of lifestyle, and the increasing importance of generics globally, the need for active pharmaceutical ingredients has skyrocketed. As a result of these, the API market growth is increasing at a rapid rate.
Based on research, the global active pharmaceutical ingredient market had an estimated value of $168.87 billion in 2019 and is presently worth $ 187.3 billion. However, the global market is expected to grow by the US $ 100 – 120 billion dollars in the next seven years to attain a market value of US $285.5 billion by 2027. The growth of the API market is expected to move at a CAGR of 6.8% during the forecast period.
This research covers growth trends and competitions in significant regions where APIs are actively manufactured and distributed. These regions include Europe, North America, Latin America, Middle and East America (MEA), and Asian Pacific (APAC). Although the majority of API manufacturing companies are located in the United States, others are located in India and China.
How APIs are synthesized?
API synthesis is a complex multi-step process that involves a wide range of chemical processes and reactions of varieties of raw materials by physical and chemical means. Specialized professionals in API manufacturing companies perform the process of active pharmaceutical ingredient synthesis and manufacturing.
APIs can be synthesized through 3 major processes – Biological process, organic chemical synthesis, and extraction from a biological source. The method used depends on the raw material involved, steps of the process, and complexity of the API molecule.
What steps are involved in the synthesis of APIs?
APIs are tiny complex molecules produced through a variety of processes. The complexity of the active pharmaceutical ingredient to be synthesized plays a significant role in determining the process used and the steps involved in the process. The steps involved in each of the three processes vary. Synthesis of APIs through biological processes include inoculum and seed preparation, fermentation, and product recovery.
Organic chemical synthesis of APIs involves the use of organic and inorganic chemicals and reactors to produce APIs. This process usually consists of the production of intermediates during chemical reactions. In some cases, these intermediates can be used to produce different APIs or used as a separate pharmaceutical entity. Extraction of APIs from a biological source involves passing natural materials through batch processes to extract or produce a pharmacologically active substance.
Although the stages involved in the various processes differ, they are all grouped into different stages - the introduction of raw materials, production of intermediates, isolation, and purification, and physical processing and packaging.
Given that technology is continually evolving, continuous flow-system has been introduced to enhance the API synthesis and manufacturing process.
How long does it take to synthesize APIs?
The length of the manufacturing process is majorly dependent on the complexity of the API molecule to be synthesized and the process used. API synthesis is a long and complicated process that may take months and sometimes, up to years to complete. The complexity of the structure influences the API synthesis period length, the number of steps in the synthesis route, type of synthesis, cost of the process, drug impurities present, number of APIs to be produced, and the number of isolation steps involved.
What is the goal behind the synthesis of APIs?
API synthesis facilitates the processing and manufacturing of pure pharmaceutical preparations (APIs) for clinical use. Through API synthesis, these active ingredients are extracted, purified, and packaged for treatment, diagnosis, cure, mitigation, and prevention of diseases. These APIs are synthesized and manufactured into biologically secure forms and substances to produce specific pharmacological effects within the recipient's body. Simply put, the primary goal of API synthesis is to create pure, potent, and safe APIs for drug development and production and administration to patients.
Challenges with API reactions and drug interactions
APIs make up the primary ingredients within drugs and are mostly responsible for drug-drug interactions. These drug-drug reactions constitute one of the major causes of failed treatment in patients as well as the production of adverse drug effects in them. Also, reactions of APIs combined within the same drug pose a significant challenge to API manufacturers and the healthcare industry.
How do API and drug interactions occur?
API and drug interactions usually occur as a result of poly-therapy, and this is mostly seen amongst elderly patients. With poly-therapy, the API of one drug interferes with the pharmacological activity of another, by enhancing or inhibiting the function of the other. These reactions usually result in diverse severe health implications and some scenarios, death. This interaction is significantly influenced by the pharmacodynamics of the body and the pharmacokinetics of the drug.
API and drug interaction can either occur during absorption, distribution, metabolism, or excretion of the drugs. Co-administration of drugs (polytherapy) greatly influences the body's response to them, and the risk of API and drug interaction rises as the number of medications administered increases. These reactions are why the API of drugs is taken into consideration when administering several drugs at once or concomitantly.
How does drug data sharing work?
The increasing need for transparency between research participants, publishers, research funders, pharmaceutical companies, regulating bodies, and trading companies during clinical trials has heightened the demand for drug data sharing. Though not yet fully established in some countries, several data sharing policies and guidelines have been set up to support this movement.
Through drug data sharing, clinical results, data, and clinical trial practices are shared and made available to pharmaceutical companies, research investigators, institutions, and other sectors to improve public health, drug development, and boost patient safety. Clinical data is shared at participant and company-level to improve company practices and public trust.
How do you set up the testing parameters of API reactions?
API testing ensures the production of stable and quality active pharmaceutical ingredients. The strength, quality, and stability of APIs are assessed by the manufacturers and regulating agencies through specific standards. Although this is done for all APIs, the testing parameters set up will vary based on the API and the brand. The primary test carried out is the stability test, and it includes stress-testing, storage conditions testing frequency, container closure system, specification, and selection of batches.
The quality of an API is directly proportional to the potency, efficacy, and safety of the medication. Low quality and compromised APIs harm the recipient's health. To ensure the production of high-quality and stable APIs, pharmaceutical companies are only allowed to handle one or two stages of the drug production process. Also, outsourced APIs and locally produced APIs and medications are thoroughly screened and tested to ensure the quality and safety of drugs.
What role does an API manufacturer play in pharmaceutical research?
The API manufacturers constitute a significant part of the pharmaceutical company globally, as well as the healthcare industry. They help to improve and facilitate pharmaceutical research in many ways. API manufacturers play a significant role in pharmaceutical research by conducting research and providing fact-based data and results on APIs and their pharmacological effects on the body.
Through in-depth analysis and a combination of efforts and contributions from active pharmaceutical ingredients manufacturers and other professionals in the pharmaceutical industry, potent and safer drugs can be developed to improve healthcare delivery.
What are the key market trends in API manufacturing?
The global demand for active pharmaceutical ingredients is on the rise, and there are a variety of factors that have actively contributed to its growth. Some of the key trends driving the development of the global active pharmaceutical ingredient market and API manufacturing include the worldwide rise of chronic diseases as well as the increasing demand for and importance of generics. Over the years, there's been a growing prevalence of chronic diseases such as chronic obstructive pulmonary disease (COPD), arthritis, hepatitis, cancer, etc., in various parts of the world.
The rapid rise of these diseases can be attributed to the increase in the geriatric population across the world. With this population experiencing a lifestyle change, and dietary consumption as a result of urbanization, an increase in the occurrence of chronic diseases such as cancer, is expected. This, in turn, will affect demand, as well as positively influence the market.
Also, as a result of technological advancement in active pharmaceutical ingredient manufacturing, the global API market is taking a huge turn around. Besides, another key trend that has positively influenced the market is the increased use of artificial intelligence-based drug discovery and production.
Market trends that have negatively influenced the global API manufacturing market include the cost of drug monitoring in other countries, intense competition between API manufacturers, and increasing production of anti-counterfeit drugs. The major market trend that has negatively influenced the market is the high cost of manufacturing APIs and unfavourable drug pricing control policies in countries across the world.
What are the key players in the active pharmaceutical ingredients market?
There are several competitor pharmaceutical companies working in the global active pharmaceutical ingredients market. These API manufacturing companies have contributed a great deal to the growth of the global API market. Some of the prominent and leading API companies include:
- Pfizer Inc. (US),
- TAPI (Teva Active Pharmaceutical Ingredients Israel),
- Sun Pharmaceutical Industries Ltd. (India),
- Aurobindo (India),
- Novartis International AG (Switzerland),
- Boehringer Ingelheim (Germany),
- Abbott (US),
- AbbVie (US),
- GlaxoSmithKline Plc (UK),
- Merck &Co. Inc. (US) amongst many others.
Regardless of these strategies, these key players in the global API market are still likely to face tough competition from new and upcoming companies in the industry.
References
Upcoming Events
-
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025 -
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025 -
CPHI & PMEC China 2025
Shanghai New International Expo Center
24 Jun 2025 - 26 Jun 2025
Pharmaceutical Industry Webinars
-
Webinar An Untapped Market in Gender Inclusivity and Equity
-
19th March 2025
-
3pm GMT /4pm CET
-
-
Webinar Pathway to $10/g Biologics Production: Reshaping the Biomanufacturing Landscape
-
18th February 2025
-
3pm BST /4pm CET
-
-
Webinar Leveraging Real-Time Clinical Data to Deliver Certainty in Solubility Enhancement and Modified Release Development
-
12th December 2024
-
3pm BST/ 4pm CET
-
-
Webinar The CPHI Sustainability Collective: A New Initiative to Support a Sustainable Pharma Value Chain
-
10th December 2024
-
3pm BST /4pm CET
-
-
Webinar Key to Success: A CDMO's Pathway to Biologics Excellence
-
5th November 2024
-
3pm BST/ 4pm CET
-
-
Webinar The Changing Dynamics of Global API Manufacturing
-
17th September 2024
-
3pm BST/ 4pm CET
-
-
Webinar Shaping the Future of Italy’s Pharma Market: Trends and Opportunities
-
4th September 2024
-
4pm CET/ 10am EST
-
-
Webinar Fragment-Based Oligonucleotide and Oligopeptide Synthesis
-
30th Jul 2023
-
4pm CET / 10am EST
-
-
Webinar GMP Rationale for Sterile High-Potency/Toxic Pharmaceuticals
-
18th June 2024
-
4pm CET / 10am EST
-
-
Webinar Unlocking Opportunities in the Growing Pharma Landscape of The Middle East
-
5th June 2024
-
3pm CET / 9am EST
-
-
Webinar Exploring Technological Trends in the Future of Pharmaceutical Manufacturing
-
23rd May 2024
-
4pm CET / 10am EST
-
-
Webinar Achieving Manufacturing Excellence Through Digital Transformation
-
16th April 2024
-
4pm CET / 10am EST
-
-
Webinar Made in Africa: What’s Driving Pharma Manufacturing
-
28th March 2024
-
4pm CET / 10am EST
-
-
Webinar Case Study: Risk Management for Annex 1 Sterile Production EMS
-
28th February 2024
-
4pm CET / 10am EST
-
-
Webinar Innovative Strategies for B2B Pharma Marketeers: Driving Value through Content
-
20th February 2024
-
4pm CET / 10am EST
-
-
Webinar Revolutionizing Pharma: Data and AI Unleashed
-
18th January 2024
-
4pm CET / 10am EST
-
-
Webinar Optimal Temperature: Elevating Biologics Cold Chain Excellence
-
16th January 2024
-
4pm CET / 10am EST
-
-
Webinar Market Outlook – The Biggest Pharma Trends of 2024
-
12th December 2023
-
4pm CET / 10am EST
-
Recommended Products And News
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance
















































































































































































































































































































.png)



.png)







.png)

.png)



.jpg)
