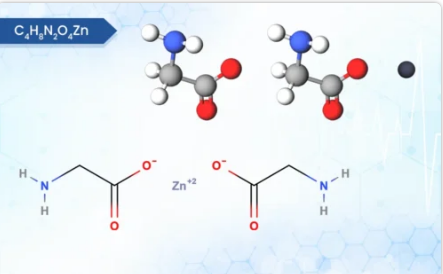
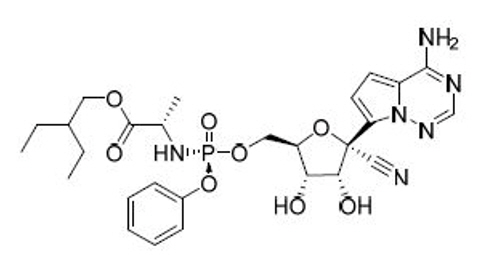
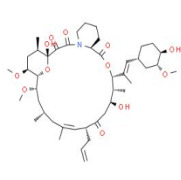
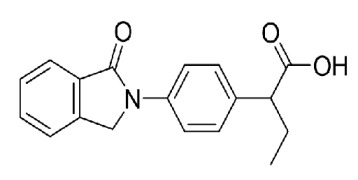
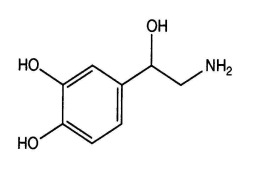
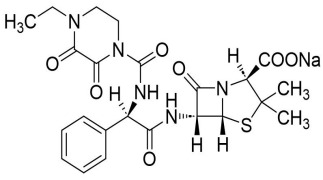
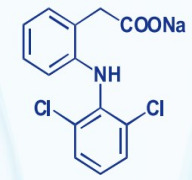
Solifenacin Succinate, Ibandronate Sodium, Sodium Alendronate, Zoledronic Acid, Brinzolamide, Dorzolamide HCl, DOTA (Tetraxetan), Gadobutrol and Gadoterate Meglumine.
Product Description
Ultratech India Limited

-
IN
-
2015On CPHI since
-
2Certificates
-
100 - 249Employees
Company types
Primary activities
Categories
Specifications
Ultratech India Limited

-
IN
-
2015On CPHI since
-
2Certificates
-
100 - 249Employees
Company types
Primary activities
More Products from Ultratech India Limited (75)
-
Product Tramadol HCL
Ultratech India Limited manufactures a wide range of API's, Advanced Drug Intermediates and Finished Formulations which includes the API of Tramadol HCL at its EU GMP approved manufacturing plant. Please refer our complete product list at www.ultratechindia.com . Visit us at CPHI -Mumbai Stand No: A ... -
Product Vardenafil Hydrochloride Trihydrate EP
Ultratech India Limited manufactures a wide range of API's, Advanced Drug Intermediates and Finished Formulations which includes the API of Vardenafil Hydrochloride Trihydrate EP at its EU GMP approved manufacturing plant. Please refer our complete product list at www.ultratechindia.com . Visit us at... -
Product Amlodipine Besilate USP/BP/EP/IP
Ultratech India Limited manufactures a wide range of API's, Advanced Drug Intermediates and Finished Formulations which includes the API of Amlodipine Besilate USP/BP/EP/IP at its EU GMP approved manufacturing plant. Please refer our complete product list at www.ultratechindia.com . Visit us at CPHI -Mumba... -
Product Amlodipine Bs., Dabigatran Etexilate Mesylate, Enalapril Maleate, Ezetimibe, Lercanidipine, Nebivolol, Rivaroxaban, Trimetazidine, Fluconazole, Itraconazole, Ketoconazole, Voriconazole, Bilastine, Cetirizine, Desloratadine, Levocetirizine
Ultratech India Limited manufactures a wide range of API's. Please refer our complete product list at www.ultratechindia.com.
Ultratech India Limited is a 30 year old transnational company specializing in the manufacturing of Active Pharmaceutical Ingredients (APIs) and ... -
Product Dolutegravir Sodium, Oseltamivir Phosphate, Valacyclovir, Valganciclovir, Budesonide, Fenoterol HBr, Fluticasone Propionate, Fluticasone Furoate, Formoterol Fumarate, Ipratropium Bromide, Montelukast, Salbutamol, Salmeterol Xinafoate, Tiotropium Bromide
Ultratech India Limited is a 30 year old transnational company specializing in the manufacturing of Active Pharmaceutical Ingredients (APIs) and a diverse range of branded generic Finished Dosage Forms (FDFs) at its cGMP certified state-of-the-art manufacturing facilities in India, with a global presen... -
Product Duloxetine, Vigabatrin, Vortioxetine, Zonisamide, Avanafil, Dapoxetine, Sildenafil Citrate, Tadalafil, Vardenafil, Glibenclamide, Linagliptin, Lansoprazole API/Pellet, Omeprazole API/Pellet, Vonoprazan Fumarate
Ultratech India Limited is a 30 year old transnational company specializing in the manufacturing of Active Pharmaceutical Ingredients (APIs) and a diverse range of branded generic Finished Dosage Forms (FDFs) at its cGMP certified state-of-the-art manufacturing facilities in India, with a global presen... -
Product Vonoprazan Fumarate
Ultratech India Limited manufactures a wide range of API's. Please refer our complete product list at www.ultratechindia.com.
Amlodipine Besilate, Dabigatran Etexilate Mesylate, Enalapril Maleate, Ezetimibe, Lercanidipine HCl, Nebivolol HCl, Rivaroxaban, Rosuvastatin Calcium, Trimetazidine H... -
Product Risedronate Sodium Hemipentahydrate / Monohydrate USP
Ultratech India Limited manufactures a wide range of API's, Advanced Drug Intermediates and Finished Formulations which includes the API of Risedronate Sodium Hemipentahydrate / Monohydrate USP at its EU GMP approved manufacturing plant. Please refer our complete product list at www.ultratechindia.com . Vi... -
Product Sodium Alendronate USP/BP/EP
Ultratech India Limited manufactures a wide range of API's, Advanced Drug Intermediates and Finished Formulations which includes the API of Sodium Alendronate USP/BP/EP at its EU GMP approved manufacturing plant. Please refer our complete product list at www.ultratechindia.com . Visit us at CPHI -Mum... -
Product Zoledronic Acid IP
Ultratech India Limited manufactures a wide range of API's, Advanced Drug Intermediates and Finished Formulations which includes the API of Zoledronic Acid IP at its EU GMP approved manufacturing plant. Please refer our complete product list at www.ultratechindia.com . Visit us at CPHI -Mumbai Stand ... -
Product Fluconazole USP/BP/EP/IP
Ultratech India Limited manufactures a wide range of API's, Advanced Drug Intermediates and Finished Formulations which includes the API of Fluconazole USP/BP/EP/IP at its EU GMP approved manufacturing plant. Please refer our complete product list at www.ultratechindia.com . Visit us at CPHI -Mumbai ... -
Product Beclomethasone Dipropionate 100 mcg Metered Dose Inhaler- BESTHALE BD 100
Ultratech India Limited manufactures a wide range of API's, Advanced Drug Intermediates and Finished Formulations which includes the Finished Formulation of Beclomethasone Dipropionate 100 mcg Metered Dose Inhalers under the Brand Name Best Hale BD-100 . Please refer our complete product list at www....
Ultratech India Limited resources (4)
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance