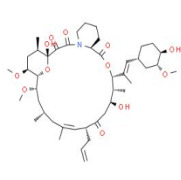
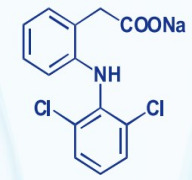
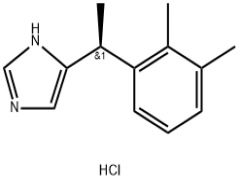
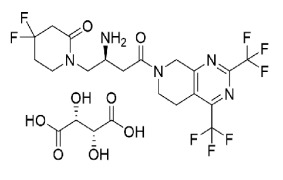
Apalutamide
Product Description
Shandong Chenghui Shuangda Pharmaceutical

-
CN
-
2023On CPHI since
-
3Certificates
-
500 - 999Employees
Company types
Primary activities
Categories
Specifications
Shandong Chenghui Shuangda Pharmaceutical

-
CN
-
2023On CPHI since
-
3Certificates
-
500 - 999Employees
Company types
Primary activities
More Products from Shandong Chenghui Shuangda Pharmaceutical (4)
-
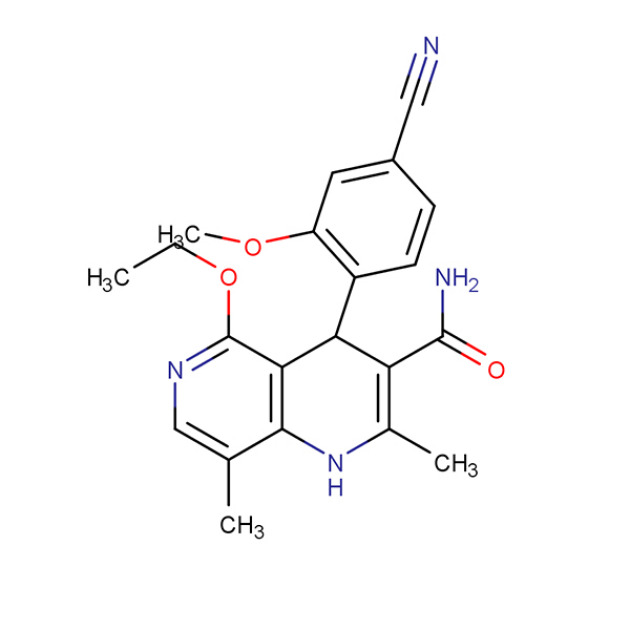
Product Finerenone
Product name: Finerenone
Alias: Finerenone (BAY94-8862); (4S)-4-(4-cyano-2-methoxyphenyl)-5-ethoxy-2,8-dimethyl-1,4-dihydro-1,6-naphthyridine-3-carboxamide; (4S)-4-(4-cyano-2-methoxyphenyl)-5-ethoxy-2,8-dimethyl-1,4-dihydro-1,6-naphthyridine-3-carboxamide; BAY94-8862; BAY948862; 1,6-Naphthyridine-3-... -
Product Resmetirom
product name: Resmetirom
Alias: MGL-3196; 920509-32-6; VIA-3196; 2-(3,5-dichloro-4-((5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yl)oxy)phenyl)-3,5- dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile; MGL 3196; Resmetirom [USAN]; MGL3196; RE0V0T1ES0
CAS No. 920509-32-6
... -
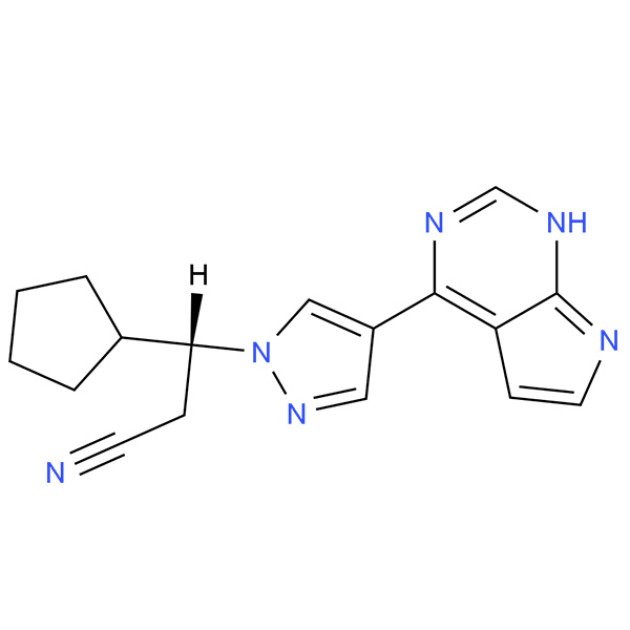
Product Ruxolitinib
Product name: Ruxolitinib phosphate
Synonyms: (R)-3-(4-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile; (betaR)-beta-Cyclopentyl-4-(7H- pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazole-1-propanenitrile; (3R)-3-(4-(7H-pyrrolo[2,3-d]pyriMidin-4-yl)-1H-pyrazo... -
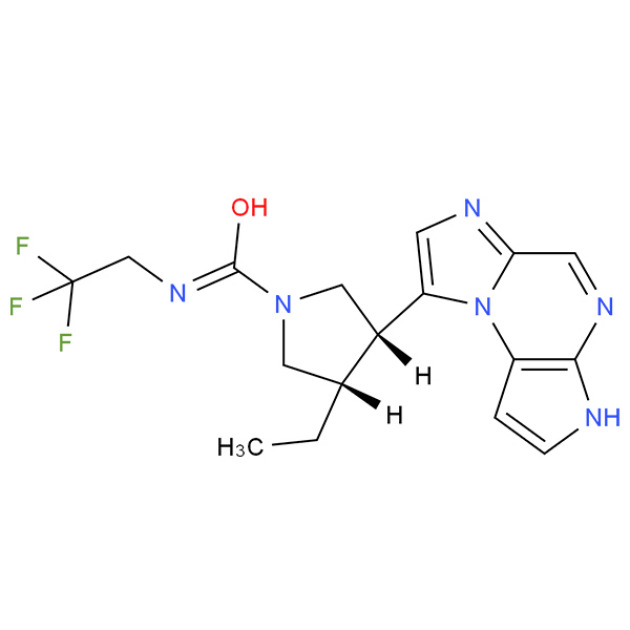
Product Upadacitinib
Product name: Upadacitinib
English Synonyms: Upadacitinib; ABT-494; ABT-494(Upadacitinib) free base; Upadacitinib (ABT-494); ABT-494 (Upadacitinib); Upadacitinib (Rinvoq); ABT-494. ABT494; ABT 494; CS-2730
CAS No.: 1310726-60-3
Molecular Formula: C17H19F3N6O
...
Shandong Chenghui Shuangda Pharmaceutical resources (2)
-
Brochure About us
Established in 2014, Shandong Chenghui Shuangda Pharmaceutical Co., Ltd. is a leading Chinese manufacturer of active pharmaceutical ingredients (APIs) and advanced intermediates. The company operates 6 cGMP-compliant workshops and 9 automated production lines, delivering a monthly production capacity of 100 metric tons. To date, it has filed 22 products with the Center for Drug Evaluation (CDE) in China, registered 4 APIs in the United States Drug Master File (US-DMF), and submitted 7 applications for Certificates of Suitability (CEP) with the European Directorate for the Quality of Medicines (EDQM).Supported by over 100 patents encompassing advanced synthesis and crystallization technologies, the company specializes in API manufacturing and offers integrated CDMO (Contract Development and Manufacturing Organization) and CRO (Contract Research Organization) collaborations.
-
Brochure Product List
The main production and R & D varieties of local anesthesia, antiviral drugs, cardiovascular and cerebral vascular system, antitumor drugs, digestive drugs, diabetes, veterinary drugs, CDMO products.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance