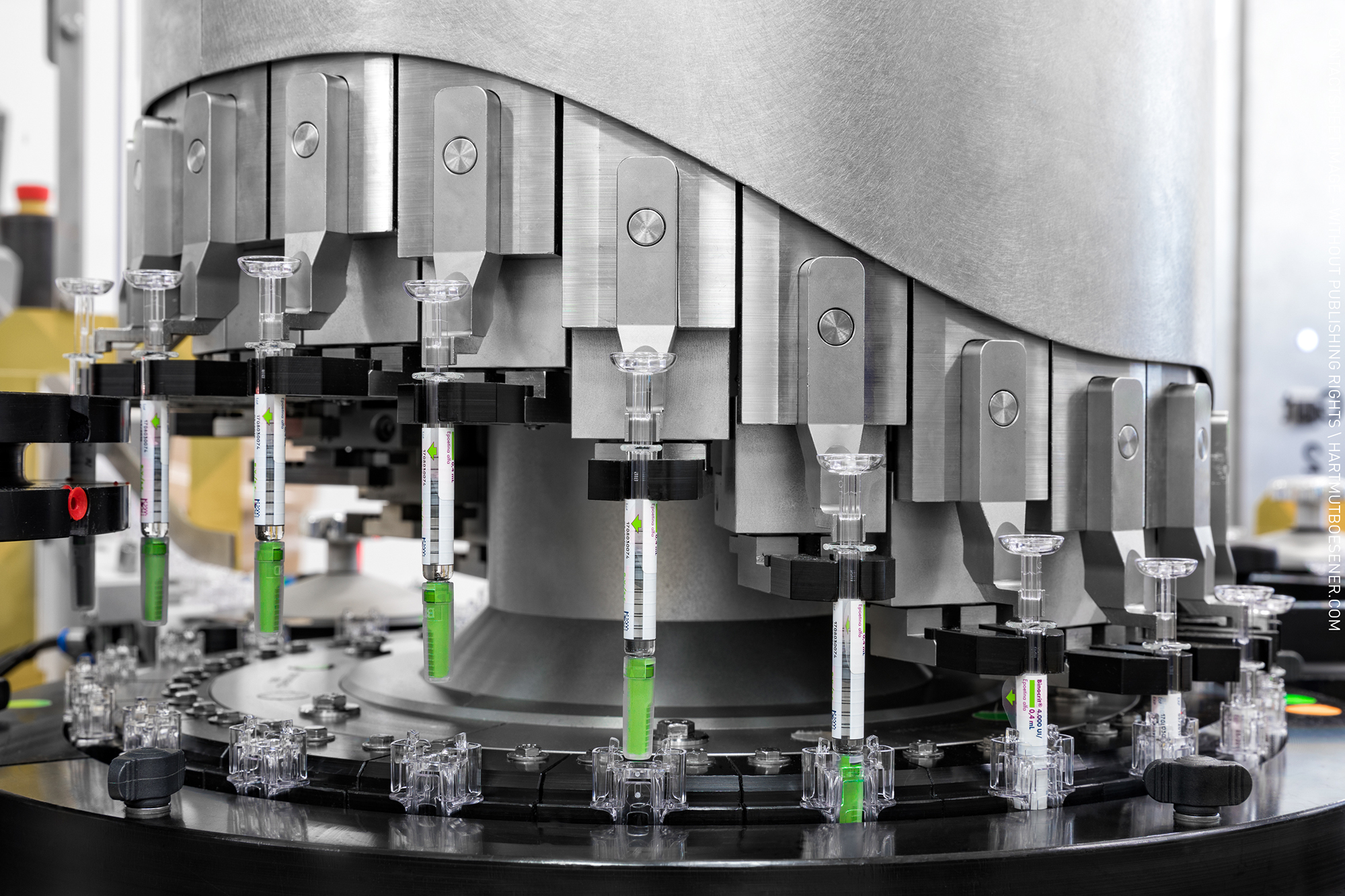
Sterile Dosage Filling and Lyophilization

Product Description
IDT Biologika GmbH

-
DE
-
2015On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
Categories
Specifications
IDT Biologika GmbH

-
DE
-
2015On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
More Products from IDT Biologika GmbH (4)
-
Product Drug Substance Manufacturing for Viral Vaccines, Gene & Immune Therapeutics, Oncolytic Viruses and Viral Vectors
Drug Substance Manufacturing for pre-clinical, clinical and commercial supply- Validation of aseptic processes - Cell and virus banking - Using own seed cell banks: VERO and DF-1, Duck cell line (AGE-1) - API production using different TechnologiesMVA Know-How IDT Biologika is considered the global lea... -
Product IDT Biologika as a Contract Development and Manufacturing Organization
IDT Biologika is an international leader in the contract development and manufacturing of biologics. The company focuses on the customized development and manufacturing of viral vaccines (phases I, II, III), gene therapeutics, immunotherapeutics, oncolytic viruses as well as sterile liquid and lyophilized ... -
Product Packaging, Quality Control, Storage, Cold Chain
Within the contract development and manufacturing, IDT Biologika GmbH provides a wide range of other pharmaceutical services for our clients products, which include- Labeling & Packaging of vials, prefilled syringes, autoinjectors - Serializiation (Track & Trace) - Quality Systems - Cold Chain up to - 80- ... -
Product Process Development for Production of Viral Vaccines and Vectors
Process Development for API Production, Fill/Finish and Secondary Packaging- Cell line development - Transfer of customer technologies -Upstream / Downstream -iCellis Fermenter: development of scalable upstream and downstream technologies for adherent cells with higher yield.Major advantage for devel...
IDT Biologika GmbH resources (3)
-
News Are you looking for Drug Substance, Fill&Finish and Packaging capacities?
We are very proud to extend our facilities in Drug Substance according to BSL 3 and to set up another high-speed filling capacities. Due to our great know-how, commercial excellence, strong partnerships and customer centricity we will push both, innovation and new production capacities for important vaccines against infectious diseases affecting people worldwide. -
Brochure About IDT Biologika Services
IDT Biologika is a globally operating biopharmaceutical CDMO that specializes inthe Contract Development and Manufacturing of Vaccines, Gene & ImmuneTherapeutics and the Fill-Finish of Biologics and other Sterile Injectables.Our mission is to accelerate the development and flexible high-quality manufacturingof our client’s therapeutic and prophylactic products, supporting ourglobal healthcare partners to treat serious diseases that impact human healthworldwide. -
News AstraZeneca partners with IDT Biologika to build COVID-19 vaccine facility in Germany
Firms plan to invest in capacity expansion at the CDMO’s manufacturing site in Dessau to boost European domestic vaccine supply
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance