Biopharmaceutical Services
Biopharmaceutical Services Companies (40)
Biopharmaceutical Services News
-
News Orbis Medicines receives funding for the development of oral biologics
Orbis Medicines, a biotechnology company based in Denmark, has recently achieved a significant milestone in its funding journey. The company has successfully secured €90 million ($94 million) in a Series A funding round, marking a substantial boos... -
News Women in Pharma: Our hopes for 2025 and beyond
Our last instalment for 2024 of the Women in Pharma series brings you messages direct from the Informa Markets CPHI team as they discuss the advice and insights they have carried throughout their roles working at CPHI, and what they hope to see for the... -
News Women in Pharma: Reflections from Behind the Scenes
In this instalment of our monthly series, the team that brings you the Women in Pharma series each month sits down for a heart-to-heart on what the series means to them, and how they hope to continue their work in the future. -
News Scaling the Industry: CPHI Scale-Up Market interview with YSK Laboratories
For the first time, CPHI Milan hosted the CPHI Start-Up Market, expanding support for emerging and small-sized enterprises in their transition to the next level of growth. In this interview, we spoke with Yuvansh Khokhani, Managing Director of YSK Labo...
Biopharmaceutical Services Products (72)
-
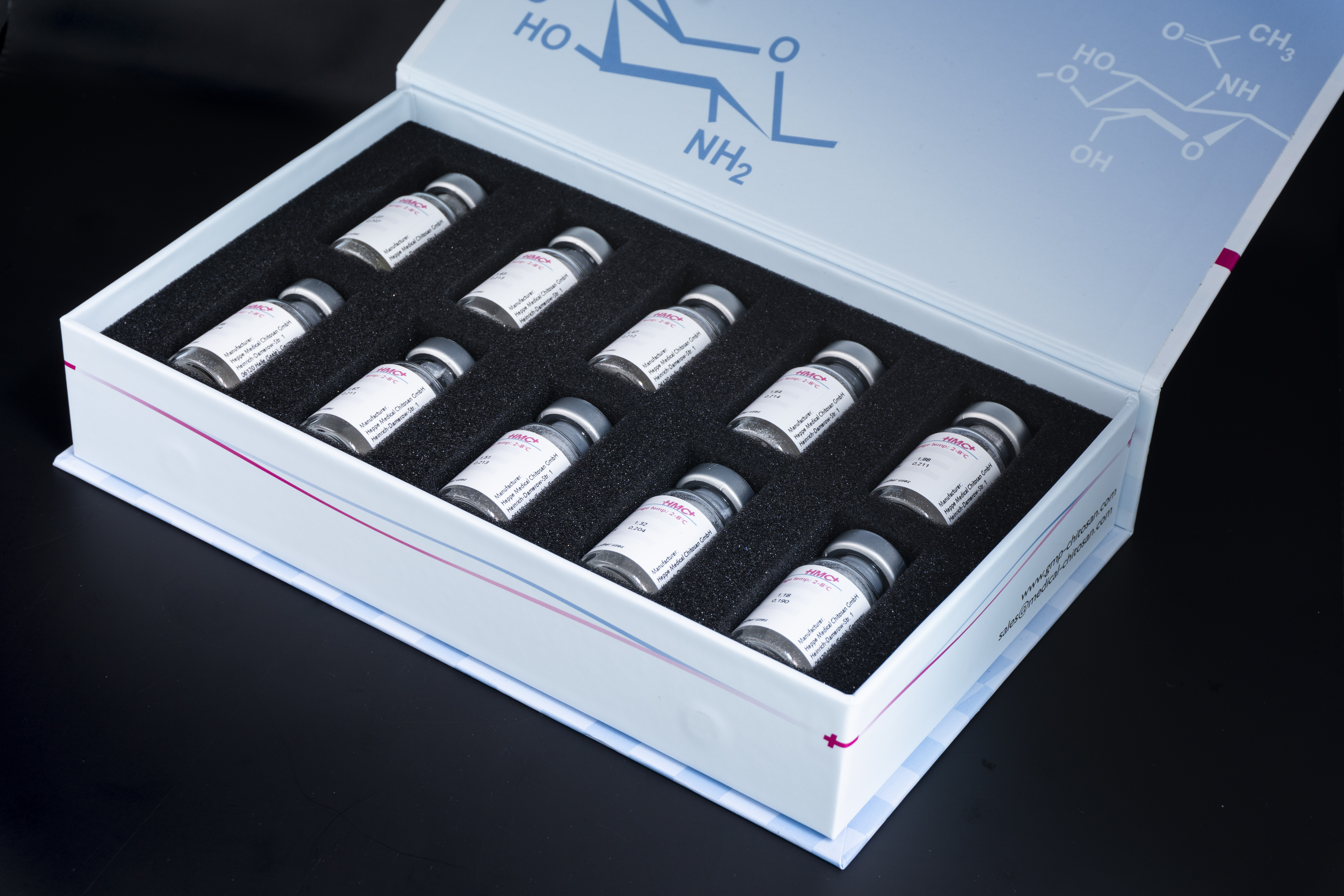
Product Biologics
End-to-end development and manufacturing capabilities across different therapeutic modalities and the entire product lifecycle
Cutting-edge technology platforms for high yielding cell lines (both mammalian and microbial) and high concentration formulations
Integrated and flexible drug sub...
-
Product GxP Audits and Inspection Readiness
Our auditors have real world experience supporting regulatory inspections and preparing companies for pre-approval inspections. Our team of specialists includes former regulatory agency inspectors and qualified auditors who are proficient in conducting mock inspections, internal audits, vendor and s...
-
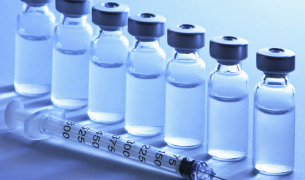
Product Sterile Dosage Filling and Lyophilization
IDT Biologika GmbH provides a wide range of pharmaceutical services which includes sterile dosage filling in vials and pre-filled syringes. With a facility size of 3,600m2, this integrated pharmaceutical/ biological site houses more than 7 filling lines for the aseptic filling of syringes, vials, ampoules ...
-
Product FLUOLEAD
Innovative nucleophilic fluorination agentBest alternative to DAST, Deoxo-fluor,... Ketone (non-enol form) and carboxylic acid can be fluorinated HIgh thermal stability Easy to handle (solid product) Only 1 step needed for deoxo-fluorination
-
Product Analytical Services
With 55+ years of experience, our expert teams develop 1,000+ analytical methods and validate 250+ methods annually. Drawing upon an extensive range of analytical technology, combined with a wealth of analytical knowledge, we can add real value to your drug development and commercialisation programs....
-
Product Biosimilars Testing Services
Testing services for biosimilars: Our biosimilar analytical comparability programs provide highly relevant data for early stage characterisation and later stage comparison. These programs evaluate and compare all pertinent features of the biosimilar product and are based on the ICH Q6B. Programs encompa...
-
Product Pharmaceutical packaging in compliance with high pharmaceutical safety standards
The main task was to integrate the different packaging formats and pack sizes of the sensitive pharmaceutical products on a compact, easy-to-operate packaging line – without losing sight of the high safety requirements typical for the pharmaceutical industry. Both 50- and 100-millilitre vials had to be pac...
-
Product Clinical Trial Services: Business Planning
Business Planning
Collaborate and build strategic plansConduct gap analysisEvaluate risks and benefitsConduct market and competitive landscape analysis
-
Product Enzyme screening & proof-of-concept studies
We offer tailor made screenings of internal and external enzyme panels to identify applicable off-the-shelf biocatalysts for your target reaction. We integrate analytical services such as method development and validation throughout the process to get a reliable statement about the feasibility of your ...
-
Product Research & Development
Discovery - Biology, Lead Optimization, Libraries, Synthetic & Medicinal ChemistryDevelopment - Chemical Process R&D, Fermentation, Formulation Development, High Potency, Kilo Lab & Small Scale Manufacturing, Lipid Nanoparticle, Method Development/Material Science, Rare/Orphan, Separation Scien...
-
Product Chemical Development Solutions
Chemical Development Solutions of GVK BIO provides a convenient, single-site distribution of infrastructure, thereby delivering a seamless and innovative transition of solutions from lab to pilot plant and bulk manufacturing, with speed and quality.
We ensure quality in the process during th...
-
Product Microbiological laboratories
BSP Pharmaceuticals S.p.A. offers a wide range of services which includes microbiological laboratories. Providing assays suitable for regulatory submission aligned with several regulatory agency requirements and equipped to carry out tests, such as: analytical methods validation, product release testing an...
-
Product POLEPHARMA BIOTESTING DAYS - EVENT
4th & 5th of February 2020
In CCI Portes de Normandie, Evreux (France)
The event will bring together 200 decision-makers and stakeholders of biotherapies’ development and production. It will last two days during which will be presented conferences, round tables, experien...
-
Product Pharmacokinetics of Therapeutic Proteins
We are fully compliance with the EMA guideline on Clinical Investigation of the Pharmacokinetics of Therapeutic Proteins and other regulations and guidelines of the FDA. Our experience covers a wide range of different kind of large molecules: • Hormones • Biosimilars: protein...
-
Product PharmaLex
PharmaLex is one of the largest providers of Development Consulting, Regulatory Affairs, Quality Management & Compliance and Pharmacovigilance worldwide. Our global teams of experts can take you through early strategic planning activities and nonclinical requirements to clinical deve...
-
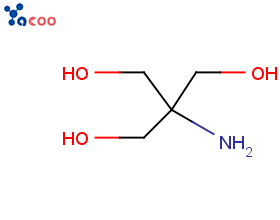
Product R-GIN (L-ARGININE 500 MG)
R-GIN (L-ARGININE 500 MG)EXPERT FERTILITY SUPPLEMENTSUPPORT HEALTHY BLOOD FLOW
-
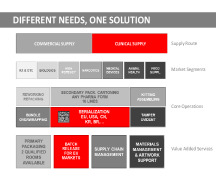
Product Outsourcing
Patheon by Thermo Fisher Scientific has a broad manufacturing platform for pharmaceutical and biologic products which provides sustainable solutions for mammalian cell-based and microbial-based manufacturing, green chemistry R&D and manufacturing technologies, and finished dosage production of biopharm...
-
Product CRO
ICBio Clinical Research, a distinguished full-service Contract Research Organization (CRO) with 16 years of experience in the industry. Based in Bengaluru, India, we specialize in delivering high-quality, integrated clinical research solutions to a diverse range of sectors including Pharmaceuticals, C...
-
Product SEMAGLUTIDE
Our main product is Semaglutide API and Injection.We manufacture Semaglutide via both synthetic and fermention routes respectively, and we also have Tirzepatide. We belong to New Hope Group, one of the world's top 500 enterprises.We are FDA-approved GM...
Upcoming Events
-
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025 -
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025 -
CPHI & PMEC China 2025
Shanghai New International Expo Center
24 Jun 2025 - 26 Jun 2025
Pharmaceutical Industry Webinars
Recommended Products And News
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance








































.png)



.png)








.jpg)
.jpg)

.jpg)





































































.png)



.png)







.png)

.png)



.jpg)











































-comp246544.jpg)




























%20(002)-comp323674.png)

















