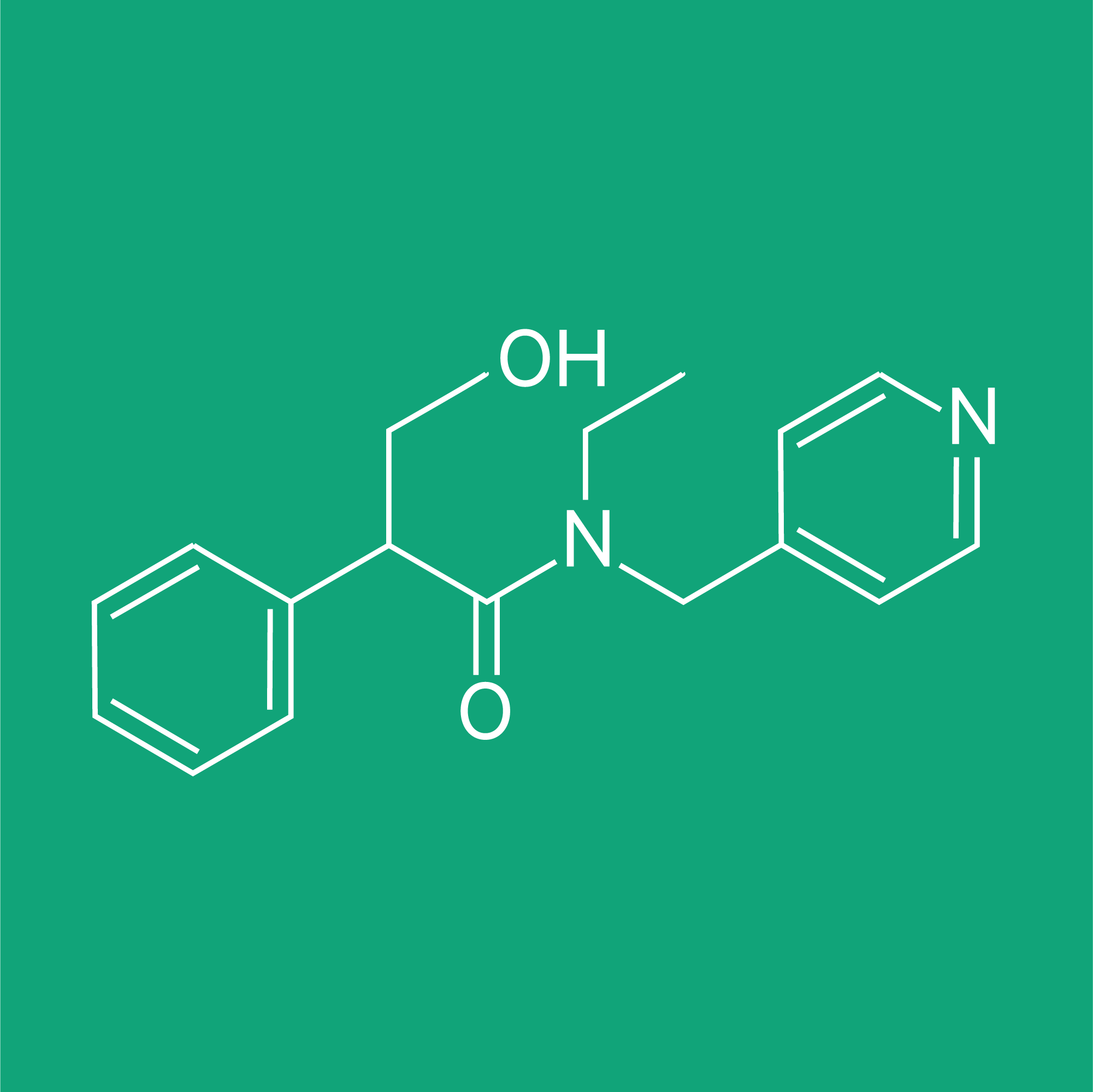
Toxicology / Virology (Biopharmaceutical Services)
Toxicology / Virology (Biopharmaceutical Services) Companies (3)
Toxicology / Virology (Biopharmaceutical Services) News
-
News QIAGEN launches world’s first syndromic test for monkeypox
The test can distinguish between monkeypox and other diseases that cause similar symptoms.
Toxicology / Virology (Biopharmaceutical Services) Products (2)
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product Permitted Daily Exposure monographs (" PDE ")
More than 1,000 API substances PDEs have already been developed in house.
New monographs are being developed on a constant basis.
Any PDE monograph can be developed upon request.
Our rates for PDE monographs are based on several factors such as status of development of the monogra...
Upcoming Events
-
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025 -
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025 -
CPHI & PMEC China 2025
Shanghai New International Expo Center
24 Jun 2025 - 26 Jun 2025
Pharmaceutical Industry Webinars
Recommended Products And News
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





.png)



.png)







.png)

.png)



.jpg)


















































































.jpg)