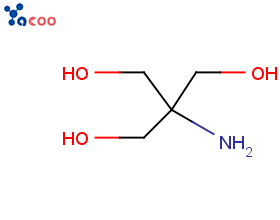
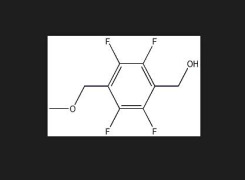
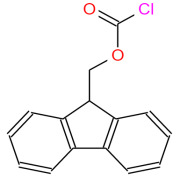
Chemical Development Solutions
Product Description
Aragen Life Sciences

-
IN
-
2019On CPHI since
-
1000 - 4999Employees
Company types
Primary activities
Categories
Specifications
Aragen Life Sciences

-
IN
-
2019On CPHI since
-
1000 - 4999Employees
Company types
Primary activities
More Products from Aragen Life Sciences (2)
-
Product Contract Manufacturing Solutions
GVK BIO offers long term Contract Manufacturing Solutions in development, validations, DMF filing, manufacturing of New Chemical Entities (NCE), Active Pharmaceutical Ingredients (API) and Intermediates.
Capabilities:Environment friendly routes under GMP conditions
Robust and cost-effective p... -
Product Formulation and Analytical Solutions
GVK BIO offers a range of Formulation R&D solutions that include pre-formulation studies, formulation development, analytical R&D, reformulation and stability studies. We can also support clinical supplies and manufacturing of exhibit batches in collaboration with our partners and offer standalone ...
Aragen Life Sciences resources (24)
-
News CPHI Pharma Award Winners 2022: CEO of the Year – Aragen Life Sciences
In this series of interviews, we speak to the teams behind the winning concepts at CPHI Pharma Awards 2022, in each of the different categories, from digital innovation to CEO of the year. -
Video Covid-19 preventive measures @ GVK BIO (Video)
With safety being our highest priority, we continue to be a reliable partner in accelerating research and development of our customers. Here’s a look at some of the precautionary measures taken against Covid19 across GVK BIO’s campuses. -
News Festival of Pharma empowers GVK BIO to meet with smaller companies that don’t normally attend events
Ahead of the world’s largest ever virtual pharma expo – the CPHI Festival of Pharma – Informa Markets spoke with Ramesh Subramanian, Chief Commercial Officer at GVK BIO on how they are preparing for the event. -
Video Contract Manufacturing Solutions (Video)
GVK BIO offers long term Contract Manufacturing Solutions in development, validations, DMF filing, manufacturing of New Chemical Entities (NCE), Active Pharmaceutical Ingredients (API) and Intermediates. -
News Oragenics Inc. and Aragen Bioscience Enter Agreement to Accelerate Development of TerraCoV2, a SARS-CoV-2 (COVID 19) Vaccine Candidate
Oragenics, Inc. (NYSE American: OGEN), (the “Company” or “Oragenics”), today announced, that through its wholly-owned subsidiary, Noachis Terra, it has entered into an agreement with Aragen Bioscience, a leading contract research organization focused on accelerating pre-clinical biologics product development, to advance TerraCov2 the Company’s SARS CoV-2 vaccine candid... -
Brochure Contract Manufacturing Solutions
GVK BIO offers long term Contract Manufacturing Solutions in development, validations, DMF filing, manufacturing of New Chemical Entities (NCE), Active Pharmaceutical Ingredients (API) and Intermediates. -
News Avid Bioservices and Aragen Bioscience Enter Agreement to Offer Biotechnology and Pharmaceutical Clients Integrated Solution for Cell Line and Process
Full Service “Sequence-to-Manufacturing” Offering Designed to Drive Efficiencies and Reduce Overall Timelines for Delivering Early-Stage CGMP Bulk Drug Substances -
Brochure Chemical Development Solutions
Small Molecule Process R&D and Manufacturing To consistently deliver value-added scientific solutions with speed and quality,while ensuring safety and compliance
Chemical Development Solutions of GVK BIO provides a convenient, single-site distribution of infrastructure, thereby delivering a seamless and innovative transition of solutions from lab to pilot plant and bulk manufacturing, with speed and quality.
We ensure quality in the process during the design and development phase by understanding the Critical Process Parameters (CPP) and Critical Material Attributes (CMA).
-
News Aragen Bioscience, Inc. and University of Technology, Sydney Enter Into MoU to Support and Accelerate Biologics R&D in Australia
Aragen Bioscience, Inc, a fully owned subsidiary of GVK BIO and a leading large molecule CRO, and University of Technology Sydney (UTS), the top-ranked young university in Australia, are pleased to announce a collaboration to accelerate biologics R&D in Australia. -
Brochure Discovery Chemistry Solutions
To consistently deliver value-added chemistry solutions, with speed and quality, while ensuring safety and compliance
GVK BIO offers fully integrated and standalone chemistry solutions from hit identification, hit to lead,lead optimization, through development for pharmaceutical, biotechnology industries and academic institutions. -
News Aragen Bioscience announces the appointment of Steven Lang, PHD, MBA as Vice President, Biologics
Aragen Bioscience is pleased to announce the appointment of Dr. Steven Lang as Vice President, Biologics. Aragen, a wholly-owned subsidiary of GVK BIO, is a leading Contract Research Organization focused on accelerating biologics product development with an integrated offering that includes antibody discovery and humanization, stable cell line development, recombinant protein production and purification, and differentiated diseases models. -
News Serum Institute and Aragen Bioscience Announce Collaboration on Vaccine Development
Aragen Bioscience, Inc, a subsidiary unit of GVK BIO and one of the leading pre-clinical CRO specializing in monoclonal antibody and other large-molecule product development and Serum Institute of India Pvt. Ltd. (Serum), the world’s largest vaccine manufacturer, are pleased to announce a collaboration for the development of multiple stable cell lines to support Serum’s HIV program. -
Brochure Peptide Chemistry
A dedicated peptide chemistry team is specialized in the synthesis of linear, cyclic, staple and modified peptides using a solution, solid or hybrid technology platforms. Our excellent infrastructure and facilities allow to synthesize diverse peptides in short time with high purities.
-
News GVK BIO Announces the Promotion of Sudhir Kumar Singh to Chief Operating Officer and Appointment of Ramesh Subramanian as its New Chief Commercial Officer
GVK BIO, a leading global Contract Research and Development Organisation (CRDO), is pleased to announce the promotion of Sudhir Kumar Singh, Ph.D., to the position of Chief Operating Officer and the appointment of Ramesh Subramanian, Ph.D., as its new Chief Commercial Officer. -
News Biological E and GVK BIO announce strategic R&D Partnership
GVK BIO, a leading global Contract Research and Development Organisation (CRDO), and BE Pharmaceuticals Inc (BE Inc), a rapidly growing pharmaceutical company that develops, manufactures and markets complex and critical injectable products, announced a strategic collaboration in drug product development. -
Brochure Formulation and Analytical Solutions
GVK BIO offers a range of Formulation R&D solutionsthat include pre-formulation studies, formulationdevelopment, analytical R&D, reformulation andstability studies. We can also support clinical suppliesand manufacturing of exhibit batches in collaborationwith our partners and offer standalone analyticalsolutions for third party formulation products. Inaddition, we offer regulatory and IP services throughour collaboration with third party consultants. -
News GVK BIO Announces Appointment of Robert R. Ruffolo, Ph.D., to its Board of Directors
GVK BIO is pleased to announce the appointment of Dr. Robert (Bob) R. Ruffolo, to its Board of Directors. -
Video From Late Pre-clinical to Commercial – Balancing Time and Cost Sensitivities
Navigating the transition from late pre-clinical stages to commercial success in the biotech and pharmaceutical industries requires a delicate balance between speed and cost efficiency. This session will explore strategic approaches to streamline this critical phase, ensuring timely market entry while controlling cost. It will deep diver into the best practices for managing late-stage development, overcoming regulatory hurdles, and scaling up production. The speaker will share insights on optimizing resource allocation, leveraging innovative technologies, and fostering strategic partnerships to enhance both speed and cost-effectiveness. Attendees will gain actionable strategies to minimize time-to-market and maximize return on investment, ensuring successful commercial launches. Join us to learn how to effectively manage the complexities and sensitivities of advancing from pre-clinical trials to commercial deployment. -
News GVK BIO Announces USFDA Approval for its cGMP Analytical Services Laboratory
GVK BIO, a leading global Contract Research and Development Organisation, announced today that United States Food and Drug Administration (FDA) has approved its cGMP Analytical Service Laboratory located at IDA, Mallapur Campus, Hyderabad. The inspection was conducted between May 1 to May 03, 2019 and the audit was concluded with NIL 483 observations. -
News LifeArc And GVK BIO Celebrate A Decade Of Partnership, Transforming Research Into Life-Saving Medicines
GVK BIO, a leading global Contract Research and Development Organisation (CRDO), and LifeArc, one of UK’s top medical research charities, are pleased to announce -
News GVK BIO announces the appointment of Axel Schleyer, Ph.D. as Chief Executive Officer, Aragen Bioscience, Inc.
May 08, 2019, Hyderabad, India/California, US: GVK BIO is pleased to announce the appointment of Dr. Axel Schleyer as Chief Executive Officer, Aragen Bioscience Inc.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance








.png)
































.jpg)