Biochemical laboratories

Product Description
BSP Pharmaceuticals

-
IT
-
2015On CPHI since
Categories
Specifications
BSP Pharmaceuticals

-
IT
-
2015On CPHI since
More Products from BSP Pharmaceuticals (8)
-
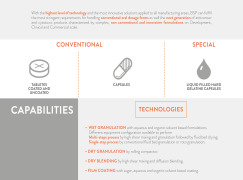
Product Oral capabilities
With the highest level of technology and the most innovative solutions applied to all manufacturing areas, BSP can fulfill
the most stringent requirements for handling conventional oral dosage forms as well the next generation of anticancer
and cytotoxic products characterized by complex, non con... -
Product ADCs Manufacturing services
BSP PHARMACEUTICALS IS SPECIALIZED TO SUPPORT THE MANUFACTURING OF ADC DRUG PRODUCTS FROM THE CONJUGATION TO THE FILL FINISH. Manufacturing lines, process flows as well as storage capabilities have been specially designed taking into consideration the critical proce... -
Product Quality control laboratories services
BSP Pharmaceuticals S.p.A. offers a wide range of services which includes quality control laboratories services. The facility houses chemical, biochemical and microbiological laboratories for the following typologies of analyses: analytical methods transfer/validation; product release testing and stability... -
Product Development services
BSP Pharmaceuticals S.p.A. offers a wide range of services which includes development services. It is conveniently situated in the production area of the plant. The specialists have the knowledge and training on the plant equipment and production requirements to reduce the risks of scale up failure. The se... -
Product Chemical laboratories quality control services
BSP Pharmaceuticals Srl offers a wide range of services which includes chemical laboratories quality control services. It includes analytical methods transfer, ich compliant method validation, in process, product release and stability testing, cleaning methods development and validation, raw materials test... -
Product Microbiological laboratories
BSP Pharmaceuticals S.p.A. offers a wide range of services which includes microbiological laboratories. Providing assays suitable for regulatory submission aligned with several regulatory agency requirements and equipped to carry out tests, such as: analytical methods validation, product release testing an... -
Product Clinical and Commercial supply services
BSP Pharmaceuticals S.p.A. offers a wide range of services which includes clinical and commercial supply services. It offers a high level of expertise to support the manufacturing of clinical products from phase I to phase III; prepare and complete PPQ manufacturing and support commercial product for world... -
Product Sterile suites capabilities
CLINICAL AND COMMERCIAL MANUFACTURING
A UNIQUE MANUFACTURING SYSTEM SPECIALLY DESIGNED FOR ANTICANCER DRUGS, WITH THE FLEXIBILITY TO MANAGE SMALL MOLECULES AND THE COMPLEXITY OF ADCs
BSP Pharmaceuticals resources (16)
-
Brochure Brochure Corporate
OUR MISSION STATEMENT
A VALUABLE AND TRUSTED NEIGHBOR IN THE ONCOLOGY COMMUNITY, OFFERING THE BEST TECHNOLOGY AND PROVEN EXPERIENCE TO INNOVATORS AT THE FOREFRONT OF THE FIGHT AGAINST CANCER.
THE FIGHT AGAINST CANCER IS OURS TOO
Every day our experience and technology are put to the service of all those forefront of the fight against cancer with determination and innovation FACED WITH SUCH A TOUGH CHALLENGE, ONLY REAL DEDICATION IS CALLED FOR The contribution of BSP to the research and development of drugs for the treatment of serious pathologies like cancer is the driving force in striving to achieve excellence In the constant evolution of anticancer drug research, the distinctive feature of the market in which BSP operates requires high specialization and continuous updating. -
Brochure Development Services
ONE STOP SHOP IN THE SUPPLY CHAIN OF ANTICANCER PRODUCTS
BSP Pharmaceuticals is a Contract Development and Manufacturing Organization focused on production of anticancer and cytotoxic drugs as small molecules and ADC compounds.
BSP Pharmaceuticals provides a full range of integrated services aimed to support the entire life cycle of a product.
From the formulation and process development/optimization, through scale up/scale down studies, we can drive the product to cGMP manufacturing for clinical and commercial needs.
QC laboratories are equipped to run all analytical testing (chemical and microbiological) on raw materials
-
Video BSP Pharmaceuticals QC Laboratories
BSP Pharmaceuticals S.p.A. offers a wide range of services which includes quality control laboratories services. The facility houses chemical, biochemical and microbiological laboratories for the following typologies of analyses: analytical methods transfer/validation; product release testing and stability; cleaning controls; environmental monitoring; raw materials testing in compliance with cgmp. It includes. Our chemical laboratories quality control services includes: analytical methods transfer, ich compliant method validation, in process, product release and stability testing, cleaning methods development and validation, raw materials testing. Our microbiological laboratories provides assays suitable for regulatory submission aligned with several regulatory agency requirements and equipped to carry out tests, such as: analytical methods validation, product release testing and stability, environmental monitoring, cleaning controls, cleaning methods development ... -
Video BSP Pharmaceuticals Development Laboratories
BSP Pharmaceuticals S.p.A. offers a wide range of services which includes development services. It is conveniently situated in the production area of the plant. The specialists have the knowledge and training on the plant equipment and production requirements to reduce the risks of scale up failure. The service includes preformulation studies, dosage form selection, compatibility studies, cleanability studies, stability, formulations, process optimization and scale up, analytical method development and validation. Contact us for more information. -
Brochure Sterile Injectable Capabilities
ONE STOP SHOP IN THE SUPPLY CHAIN OF ANTICANCER PRODUCTSBSP Pharmaceuticals is a Contract Development and Manufacturing Organization focused on production of anticancer and cytotoxic drugs as small molecules and ADC compounds.
BSP Pharmaceuticals provides a full range of integrated services aimed to support the entire life cycle of a product.
From the formulation and process development/optimization, through scale up/scale down studies, we can drive the product to cGMP manufacturing for clinical and commercial needs.
QC laboratories are equipped to run all analytical testing (chemical and microbiological) on raw materials, components, in process controls, release and stability testing.
-
Video BSP Pharmaceuticals Sterile vials filling injectable
BSP Pharmaceuticals is a Contract Development and Manufacturing Organization focused on production of anticancer and cytotoxic drugs as small molecules and ADC compounds. BSP Pharmaceuticals provides a full range of integrated services aimed to support the entire life cycle of a product. From the formulation and process development/optimization, through scale up/scale down studies, we can drive the product to cGMP manufacturing for clinical and commercial needs. QC laboratories are equipped to run all analytical testing (chemical and microbiological) on raw materials, components, in process controls, release and stability testing. Internal Regulatory Affairs team to manage data collection and document preparation for DMF and CMC to support filing activities. -
Brochure Oral Dosage Capabilities
ONE STOP SHOP IN THE SUPPLY CHAIN OF ANTICANCER PRODUCTS
BSP Pharmaceuticals is a Contract Development and Manufacturing Organization focused on production of anticancer and cytotoxic drugs as small molecules and ADC compounds.
BSP Pharmaceuticals provides a full range of integrated services aimed to support the entire life cycle of a product.
From the formulation and process development/optimization, through scale up/scale down studies, we can drive the product to cGMP manufacturing for clinical and commercial needs.
QC laboratories are equipped to run all analytical testing (chemical and microbiological) on raw materials, components, in process controls, release and stability testing.
-
Brochure BSP Pharmaceuticals Oral dosage department
BSP Pharmaceuticals is a Contract Development and Manufacturing Organization focused on production of anticancer and cytotoxic drugs as small molecules and ADC compounds. BSP Pharmaceuticals provides a full range of integrated services aimed to support the entire life cycle of a product. From the formulation and process development/optimization, through scale up/scale down studies, we can drive the product to cGMP manufacturing for clinical and commercial needs. QC laboratories are equipped to run all analytical testing (chemical and microbiological) on raw materials, components, in process controls, release and stability testing. Internal Regulatory Affairs team to manage data collection and document preparation for DMF and CMC to support filing activities. -
Brochure ADCs Capabilities
ANTIBODY DRUG CONJUGATES (ADCs)
BSP Pharmaceuticals is specialized to support the manufacturing of ADC products from the Conjugation to the Fill-Finish and offers a full and integrated package of services from development to clinical and commercial supply.
Development, Scale up/down, Quality control, Manufacturing, Registration
-
Video BSP Pharmaceuticals ADCs Liposome manufacturing
BSP PHARMACEUTICALS is specialized to support the manufacturing of ADC Drug product from the conjugation to the fill finish. Manufacturing lines, process flows as well as storage capabilities have been specially designed taking into consideration the critical process parameters and the risk factors associated with the handling and production of this class of compounds. -
Brochure BSP Pharmaceuticals Sterile vials filling injectable
BSP Pharmaceuticals is a Contract Development and Manufacturing Organization focused on production of anticancer and cytotoxic drugs as small molecules and ADC compounds. BSP Pharmaceuticals provides a full range of integrated services aimed to support the entire life cycle of a product. From the formulation and process development/optimization, through scale up/scale down studies, we can drive the product to cGMP manufacturing for clinical and commercial needs. QC laboratories are equipped to run all analytical testing (chemical and microbiological) on raw materials, components, in process controls, release and stability testing. Internal Regulatory Affairs team to manage data collection and document preparation for DMF and CMC to support filing activities. -
Brochure BSP Pharmaceuticals Development Laboratories
BSP Pharmaceuticals S.p.A. offers a wide range of services which includes development services. It is conveniently situated in the production area of the plant. The specialists have the knowledge and training on the plant equipment and production requirements to reduce the risks of scale up failure. The service includes preformulation studies, dosage form selection, compatibility studies, cleanability studies, stability, formulations, process optimization and scale up, analytical method development and validation. Contact us for more information. -
Brochure BSP Pharmaceuticals ADCs Liposome manufacturing
BSP PHARMACEUTICALS is specialized to support the manufacturing of ADC Drug product from the conjugation to the fill finish. Manufacturing lines, process flows as well as storage capabilities have been specially designed taking into consideration the critical process parameters and the risk factors associated with the handling and production of this class of compounds.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance