- Home
- Finished Dosage Forms (Products)
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
Finished Dosage Forms (Products)
Finished Dosage Forms (Products) Companies (500+)
Finished Dosage Forms (Products) News
-
News Key Insights from the Heart of Pharma: an exclusive CPHI Online report
CPHI Milan, held from October 8–10, 2024 in the historic Fiera Milano, celebrated its 35th edition of bringing together the pharmaceutical industry and supply chain for 3 days of collaborating and innovating. Our first CPHI Wrap-Up Report brings ...14 Nov 2024 -
News CPHI Milan 2024 - From the Floor
Milan and CPHI welcome you to 2024 CPHI Milan! As we celebrate the 35th edition of our flagship CPHI show, editors Vivian Xie and Lucy Chard bring you the latest from the show floor, conference sessions, and innovative solutions from all exhibitors, at...7 Oct 2024 -
News Pharmaceutical Supply Chain People Moves
The latest appointments, promotions, and structural changes across the pharmaceutical supply chain.10 Sep 2024 -
News Providing solutions for special populations – CPHI North America Interview
In Philadelphia in May on site at CPHI North America we were able to meet with some of our speakers regarding their presentations. In the following interview Srinivasan Shanmugam, Executive Director, Pharmaceutical Sciences, Business Sup...10 Jun 2024 -
News Breaking Barriers: Innovations in Oral Solid Dose Form Bioavailability
The effectiveness of a medication often hinges on its bioavailability – the rate and extent at which the active ingredient is absorbed into the bloodstream. When it comes to oral solid dose forms, such as tablets and capsules, the challenge lies ...28 Aug 2023 -
News Rebuilding the United States' pharma infrastructure – CPHI North America 2023 preview
As we prepare for CPHI North America from April 25–27 in Philadelphia, USA, CPHI Online caught up with some of the track sponsors to discuss how the show connects vital players within the North American pharmaceutical landscape. ...18 Apr 2023 -
Sponsored Content How healthcare trends inform dosage forms
Capsules encompass one of the most popular solid oral dosage forms for pharmaceutical products, with the global empty capsule market predicted to rise to USD $3.7 billion by 2026. The growth in the capsule market can be partly attributed to the many op...28 Feb 2023 -
News Ten23 health expands newly acquired sterile drug product manufacturing site
The expansion will provide additional cold storage and visual inspection capacity, as well as cleanrooms for device assembly and secondary packaging28 Mar 2022 -
News HCmed to expand inhalation treatment business with new CDMO offering
The Taiwan-based nebuliser specialist has signed an MoU with Formosa Laboratories, and Formosa Pharmaceuticals to form a biopharma one-stop-shop7 Mar 2022 -
News ANI Pharma enhances generics and CDMO business through Novitium acquisition
The transaction expands ANI’s R&D pipeline and adds nine new customers to its growing CDMO business24 Nov 2021 -
News Meiji Seika to expand CDMO business with new manufacturing facility in India
The $20.1 million investment will enable the company to manufacture pharma products for the Adcock Ingram Group and for other clients10 Nov 2021 -
News CPHI Worldwide is back, and this time it’s hybrid
A new in-person and online edition of CPHI Worldwide, transforming your event experience.1 Nov 2021 -
News Next stop, Milan - Welcome to Italy's life sciences hub
Milan is home to an internationally competitive pharmaceutical, biotechnology and functional foods hub, with strong growth prospects and a commitment to innovation across its entire supply chain. Italy is also home to one of the most advanced and ...21 Oct 2021 -
News MannKind signs sale-leaseback agreement for inhaled insulin manufacturing site
The transaction will generate $102.25 million to support the company's product pipeline and scaling up commercial activities for Afrezza1 Oct 2021 -
News Lonza to establish multi-product fill and finish line in China
The new production line at Guangzhou site will supply global and domestic companies with clinical and commercial batches23 Aug 2021 -
News Discover the CPHI North America Learning Labs: Part One
Explore the series of Learning Labs at CPHI North America across several product innovation categories in which thought leaders at our exhibitors showcase their extensive expertise in all areas of the pharma supply chain, offering industry insight...30 Jul 2021 -
News CPHI Online Report - Pharma Market Trends 2021
Twelve pharma market trends we anticipate across 2021 for the drug development, manufacturing and outsourcing sectors.25 Nov 2020 -
News Recipharm signs up for aseptic fill-finish manufacturing of Moderna COVID-19 vaccine
The CDMO is already making certain investments to enable technology transfer and scale-up to commence imminently.24 Nov 2020 -
News Vaccine manufacturing demand has had limited effect on regenerative medicine sector, panel tells CPHI audience
The ramping up of vaccine manufacturing demand amid the COVID-19 pandemic had an initial impact on cell and gene therapy production but the regenerative medicine sector has largely weathered the storm, according to senior industry figures in a recent p...26 Oct 2020
Finished Dosage Forms (Products) Products (2500+)
-
Product Capsaicin Cream
• Capsaicin is used to help relieve a certain type of pain known as neuralgia (shooting or burning pain in the nerves). • Capsaicin is also used to help relieve minor pain associated with rheumatoid arthritis or muscle sprains and strains.
-
Product Skin protectant spray
The product helps prevent and temporarily protects and helps relieve chafed, chapped or cracked skin. It helps prevent and protect from the drying effects of wind and cold weather
-
Product Tebarat 0.5 mg/ml - Allergic conjunctivitis - single-dose vials eye drops - Rx
Tebarat 0.5 mg/ml eye drops is indicated for treating and preventing allergic conjunctivitis. Treatment and prevention of seasonal allergic conjunctivitis symptoms in adults and children aged 4 years and over.Treatment of non-seasonal (perennial) allergic conjunctivitis symptoms in adults and children aged...
-
Product Norethisterone Controlled Release Tablet 10 mg - NORETHIS-NCR
No More Irregularities with Norethis
Shree Venkatesh International Ltd offers a wide range of pharmaceutical products, including Norethisterone Controlled Release Tablet 10 mg - NORETHIS-NCR.
• Use: Norethisterone is used to treat women with abnormal bleeding from the uterus. It...
-
Product Methylphenidate Hydrochloride MR
MR - Treatment for children (over 6 years old), adolescents and adults with Attention Deficit Hyperactivity Disorder (ADHD). Box containing 30 capsules of 10/20/30/40/60 mg. Other packs are also available.
Rubifen LA® is a bioequivalent generic of Ritalin LA® in the treatment of AD... -
Product DINADAX CANDY BARS - BRINGS OUT THE BEST OF YOU
Food supplement in the form of candy bar.
Dinadax ENERGY to supply energy.
COMPOSITION: Royal Jelly, Vitamin B1 and B6.
INDICATION: Exercise and sport, periods of tiredness.
FLAVOUR: Strawberry.
PRESENTATION: Box of 20 bars
Dinadax ACTIVE for the immun...
-
Product Doxorubicin-Kimia
• An anthracycline mechanism which intercalates into DNA and inhibits topoisomerase II, promoting the generation of free radicals that induce tumor cell death. • Demonstrates a longstanding, evidence-based role in treating breast cancer, lymphomas, sarcomas, and other malignancies. • Delivered via IV in...
-
Product Cogiton Long Time
Cogiton Long Time is a patented food supplement based on a specific and harmonized combination of Ginkgo Biloba, B vitamins, carnosine, coenzyme Q10, L-cysteine, beta-carotene, vitamin E, vitamin C and selenium.
Cogiton Long Time is available in capsules, it is recommended to take one capsule...
-
Product Vaginal Microflora - GYNELLA® Flora, Gynella® Baalance
Innovative Medical devices based on tyndallized lactobacili. The non-viable strains are aligned with new regulations of Medical Devices in EU.
Gynella Flora
Contains 3 lactic strains (Lactobacillus acidophilus, Lactobacillus reuteri, Lactobacillus casei) to support natural protective mechan...
-
Product Imipenem Cilastatin 500 mg + 500 mg
Imipenem Cilastatin is indicated for the treatment of serious infections caused by susceptible strains of the designated microorganisms in the conditions listed below
Lower respiratory tract infectionsUrinary tract infections (complicated and uncomplicated)Intra-abdominal infec...
-
Product Sterile Drug Product services
CARBOGEN AMCIS offers Sterile Drug Product services, specializing in the formulation, development, and manufacturing of injectable products. With capabilities in lyophilization, sterile filling, and handling highly potent APIs, the company ensures aseptic processing for clinical and commercial supply. ...
-
Product BAUSCH Consumables Ready to Use IV Bags
BAUSCH Consumables a division of BAUSCH Advanced Technology Group now offers “Ready to Use” IV Bags. • Volumes 50 ml - 1 L • Single or Dual Chamber • Pre-Printing on Bags • Multilayer Polypropylene Based Film • Sterile • Easy Twist Off Ports • Custom Sizes ...
-
Product UROKINASES SYNER MEDICA 500.000
Syner-KINASE® is indicated for the lysis of blood clots in the following conditions: - thrombosed intravascular catheters and cannulae - extensive acute proximal deep vein thrombosis - acute massive pulmonary embolism - acute occlusive peripheral arterial disease with limb threatening ischemia
-
Product Brown Spot Treatment Kit
Brown Spot Kit is a unique concept that offers professional treatment of brown spots at home. The Pen removes brown spots through a local peel whereas the Cream boosts the exfoliation process to speed up healing of the skin. Classification: Medical Device Class I Format: 2g Precision Pen + 50mL Preventio...
-
Product FDF - onoclogy , Diabetic , Anti malaria ,
general catagury API we offer API Acyclovir Deferaserox Artimether Lumfentrine www.globelapharma.com
-
Product PROBIOSPRAY
Sprayable Lactobacillus rhamnosus GG in oil. Double care: immunity and oral health for children. Efficacy clinically proven.
-
Product BENPAIN
Benpain (Benzydamine HCl) Mouthwash% 0,15; 120ml Benpain (Benzydamine HCl) Oral Spray % 0,15; 30 ml
-
Product Cicaialo Gel Wounds
Acts by protecting the wound that has damaged the derma creating an optimalenvironment for repairing and protecting processes, at the same time, the area fromexternal agents of physical (rubbing) and bacterial nature.Takes coadjuvant action in the repairing process of the tissue in the case of:- Irritation...
-
Product PROBIOTIC CAPSULES FOR WOMEN'S HEALTH
PRO-VG CAPSULES FOR WOMEN'S HEALTH ARE DESIGNED, DEVELOPED AND FORMULATED USING CLINICALLY TESTED PROBIOTIC STRAIN TO BE TAKEN ORALY, WHICH HELPS TO FIGHT VAGINAL INFECTION.
-
Product Pollen & Pollution Catcher Nasal Spray
With Moisturizing & Soothing Mallow Prevents main allergy symptoms while keeping nasal mucosa soothingly moistened
-
Product Finished Dosage Form
modified-release tablets (MUPS and matrix tablets)film coated- or dispersible tabletsprinted tabletsmodified release capsulesmicro-tablets in capsulescontrolled substances & broad-spectrum antibioticssugar coated tablets
-
Product Rontamil Mom
Rontamil Mom: All-in-one support, formulated specially for all stages as before conception, throughout pregnancy and during breast feeding.If a mother does not get adequate amounts of certain nutrients, it can decrease the nutrient levels in her milk. This is most often a problem in areas where malnutritio...
-
Product Cellusan
Farmigea S.P.A. offers wide range of pharmaceutical products which includes Cellusan. Cellusan is used to obtain long lasting and soothing relief from moderate to severe dry eyes. The product is preservative free. Contact us for more information.
-
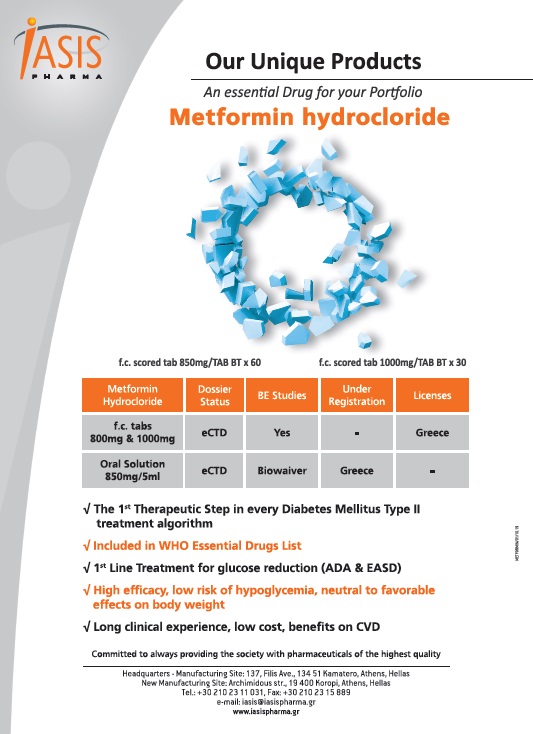
Product METFORMIN HYDROCHLORIDE
Iasis Pharma S.A. provides a wide range of products which includes Metformin Hydrochloride. 1st therapeutic step in every Diabetes Mellitus Type II treatment algorithm. 1st line treatment for glucose reduction (ADA & EASD). Contact us for more information.
-
Product Velvet Colors / Matte Make Up - NEW
Make-up for an ideal result of color coverage with a velvety, matte texture that perfects skin look. It has a special color integration technology adapted to the natural skin tone, for completely seamless and natural coverage of imperfections without a mask effect. With patented Second Skin Velvet Tech...
-
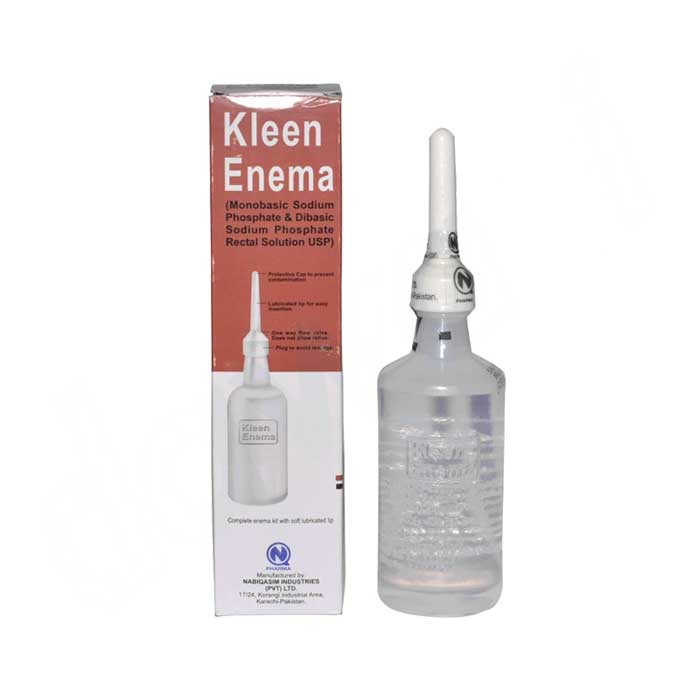
Product Kleen Enema / Laxative Enema / Monobasic Sodium / Dibasic Sodium Phosphate
Rectal Solution 135Ml
-
Product emollient sprays
Aurena laboratories AB manufactures of bag-on-valve aerosols for the pharmaceutical and medical device companies which includes emollient sprays. It belongs to wound & skin care products category. It acts as a water repellent and creates a barrier that protects and soothes irritated and intact skin from da...
-
Product EVER Pharma Product List
Our products include: Atosiban Cabazitaxel Cebrium(R) Cerebrolysin(R) Dacepton(R) Dexmedetomidine Docetaxel Fulvestrant MemoProve(R) Oxytocin Paclitaxel Pemetrexed Tachyben(R) Terlipressin
-
Product Formulation Development
Recipharm offer formulation development services for all dosage forms. We develop everything from simple formulations for early studies to more complex formulations suited for commercialisation.
-
Product Spray Dryer
GEA Niro pharmaceutical spray dryers from R&D to large production scale. Come and discuss your Pharma Spray Drying project with us or learn more about our pharma spray drying technology. Welcome!
-
Product Isotonic Nasal Cleansing Spray
With Natural Isotonic Seawater Prevents & Relieves effectively all your nasal symptoms For babies over 3 months, children & adults
-
Product Directly Compressible Granules
1. Aceclofenac Granules 50%2. Clopidogrel Granules 60% 3. Fexofenadine Granules 48% 4. Gliclazide Granules SR 48% 5. Levitiracetam Granules SR 75%, 80% 6. Lisinopril Granules 50% 7. Metformin HCL Granules IR 86.66%, 93% 8. Metformin HCL Granules SR 75%, 80% 9. Montelukast Granules 7.1% 10. Paracetamol Gran...
-
Product Grape Seed Extract - Raw material is import from France
Vivian What's App/Mob: +86 13142188019 Tel: 001-9093202087 Skype: vivianxie1314 Email: vivian@phyhuir.com Certificate of Analysis Grape Seed Extract Extract Solvent: Water ITEM SPECIFICATION RESULT STANDARD REFERENCED ...
-
Product MANIPTIN 100 (sitagliptin 100 mg.)
Sitagliptin 100 mg. film-coated tablet - stagliptin phosphate monohydrate 128.5 mg. equivalent to Sitagliptin 100 mg.
-
Product Nitrosamines - QC / Development / Validation
LC-MS/MS methods are nowadays commonly used for nitrosamines analysis in all types of materials, since it allows analyte-specific detection based on both retention time and structurally specific fragmentation information in conjunction with high sensitivity.
After the establishment of the ni...
-
Product IND Enabling Preformulation Package (IEPP)
WuXi STA's IND Enabling Pre-formulation Package (IEPP) is an integrated and end-to-end developability assessment service for new chemical entities (NCE's). Through this comprehensive service, you can efficiently identify challenges and tailored solutions earlier for higher project success rates and lower o...
-
Product Contract manufacturing services
We manufacture, develop and consult. • Product portfolio: Tablets, film-coated tablets, suppositories, cremes, gels, ointments, capsules, Packaging • State-of-the-art GMP production facilities • Manufacturing according to your individual requirements • Blister and finished packaging, s...
-
Product Cross-Linked Intra Articular Gel | VISCOSUPPLEMENT | Modified Hyaluronic Acid 2-5 mL | 10-120 mg
SEMICAL® Gel-B Cross Sodium Hyaluronate for Intra-Articular Injection
Mimics Synovial Fluid!
Advanced Thixotropic Process Makes it Unique!
SEMICAL® intra-articular Gel-B Cross is a highly purified bio-fermented sodium hyaluronate based viscoelastic solution ...
-
Product Human albumin & other Blood products
• Human Albumin IVIG • Dried Factor VIII Fraction • Erythropoietin • Darbepoetin alfa Injection • Filgrastim • Human Anti-D Immunoglobulin
-
Product Product list
ExtractumPharma Co.Ltd. is a privately owned Hungarian pharmaceutical company foundedin 1993 as a small private business. Over the past 30 years it has grown into one of the decisiveplayers of the medium-sized enterprise sector in Hungary. ExtractumPharma is a GMP approvedpharmaceutical manufacturing compa...
-
Product Forminol® - Diabetes Treatment Support
Forminol® is a cutting-edge food supplement developed through a rigorous 4-year R&D project, specifically designed to support diabetes treatment. It is particularly beneficial for individuals with type II diabetes and prediabetic patients, including those on metformin therapy. Forminol® represents...
-
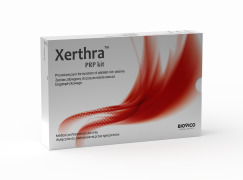
Product Xerthra™ kits
Xerthra™ PRP kit is a new generation, high-performance platelet (PLT) separation device, dedicated to preparation of platelet-rich plasma (PRP) from patient’s blood. Large number of growth factors released from PLTs delivered by PRP will lead to improvement of regenerative properties of patient’s cell...
-
Product L-carnitine solution for injection 1g/5ml
Levocarnitine solution for injection 1g/5ml is indicated for the treatment of:
Acute metabolic deregulation due to L-carnitine deficiency Secondary L-carnitine deficiency in hemodialysis patients with end-stage renal failure
L-Carnitine is a...
-
Product Hemofence Heostatic (Thrombin)
Hemostatic with Thrombin (5000IU) + Crosslinked hyaluronic acid gel
-
Product Azithromycin Tablets - Azom
Azom contains Azithromycin, is an acid-stable macrolide antibiotic that inhibits microbial protein synthesis. It prevents bacteria from growing by interfering with their protein synthesis.
Indication - It is used to treat Lower & Upper respiratory tract infections, uncomplicated skin infecti...
-
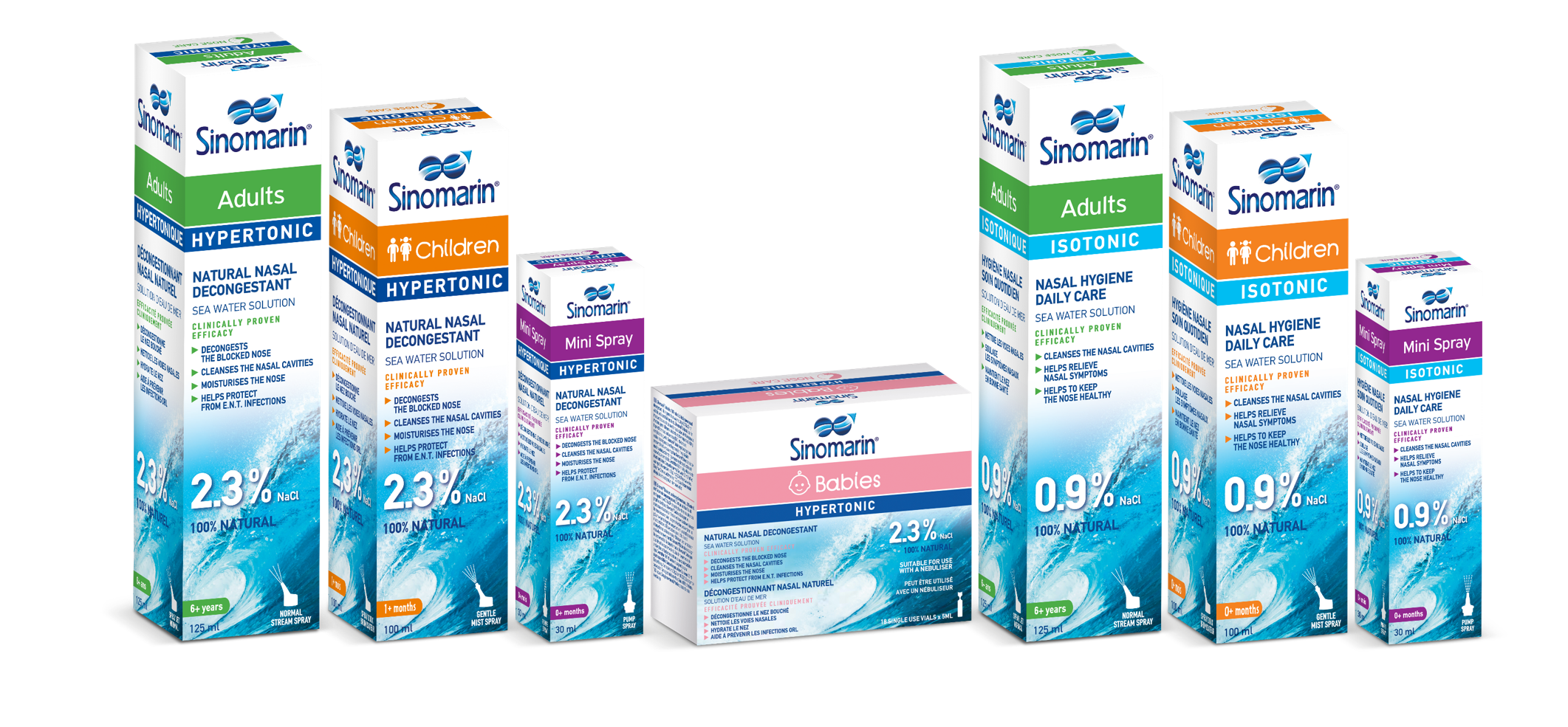
Product Sinomarin® -Seawater Nasal Sprays & Vials
Pioneered 25 years ago as the first hypertonic seawater spray for nasal care, Sinomarin is, today, a prominent global brand in natural nasal care with presence in >45 international markets. All products are Medical Devices, offering natural solutions for nasal symptom management. Sinomarin devo...
-
Product Registration Dossiers / Finished Dosage Forms
Carbocisteine CapsulesCarbocisteine Syrup
Clopidogrel Tablets
Dexketoprofen Tablets
Dimenhydrinate Tablets
Doxazosin Tablets
Fexofenadine Tablets
Folic acid Tablets
Lisinopril + HCTZ Tablets
Minoxidil Cutaneous Spray
Perindopril Tablets
Perindopril + Indapamide Tablets q... -
Product Probiositive (Mental Health)
Probiositive is a Food supplement containing probiotic live lactic acid bacteria (Probiotic blend of Lactobacillus helveticus Rosell®-52 and Bifidobacterium longum Rosell®-175) with SAM-e, Magnesium andVitamin B6. Its packaging in orosoluble sachets, with Dry-SP-SystemTM technology, makes it particularly c...
-
Product Cotrimoxazole for oral suspension
Cotrimoxazole is a sulfanilamide antimicrobial drug, which is a combination of sulfamethoxazole (SMZ) and trimethoprim (TMP). It has good antibacterial effect on non-enzymatic Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumoniae, Escherichia coli, Klebsiella, Salmonella, Proteus, Morgane...
-
Product Contract Development & Manufacturing: Oral Dosage Form
Validated End-to-End Capabilities from Product Development to GMP-approved Commercial Manufacturing of Oral Solid Dosage Forms. Bioplus supports all stages of science: Formulating, Scale-up, Manufacturing & Delivery. ioplus supports all stages of science: Formulating, Scale-up, Manufacturing ...
-
Product Rhei Clear Plus
Rhei Clear Plus is diosmectite powder indicated for the oral treatment of acute and chronic diarrhoea. It is registered as Class IIa Medical Device.
1. Protecting the GI Mucosa and entrapping diarrhoea – causing substances2. Absorbs water from the large intestine restoring balance
qq...
-
Product Solid Dosage Forms
Solid Dosage Forms: Various tablet forms (plain, coated, controlled release), capsules, granules in sachets and jars.

-
Product Generic (Paracetamol + Thiocolchicoside )
- Active substance(s): Paracetamol + Thiocolchicoside
- Pharmaceutical form(s): Tablets
- Strength(s): 500 mg + 2 mg
-
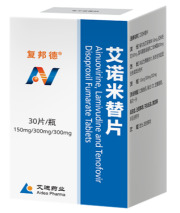
Product Ainuomiti Tablets (Anti-HIV/AIDS innovative drug)
Ainuomiti Tablets
(Ainuovirine, Lamivudine and Tenofovir Disoproxil Fumarate Tablets)
The first innovative oral anti-HIV/AIDS single-tablet combination in China, containing key NCE of Ainuovirine.

-
Product Propofol Injection 10 mg/ml (1% w/v)
Indication:
For induction & maintenance of anesthesia
Available as:
10 mg/ml (20 ml & 50 ml vial)

-
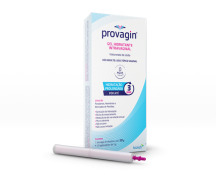
Product Women's Health - Provagin® & Higicalm®
=> PROVAGIN® (HA) Vaginal gel is indicated for vaginal hydration as a soothing and restorative agent. In addition to its hydrating property, it may also help improve vaginal secretions, elasticity and the maintenance of the physiological vaginal pH - It is not a lubricant only.
Available ...
-
Product Easyfishoil Multi
EasyFishoil Multi
Fishoil supplement including multivitamins with EPA and DHA
Easy-to-chew gellies
Natural strawberry and lemon flavour
No added sugars and no artificial additives
Blister pack protects against air and moisture, so a fresh product every day.
-
Product Disease Activity Models/Tissue Culture
MedPharm has developed proprietary models relevant for major disease areas such as psoriasis, atopic dermatitis, onychomycosis and continue to develop new ones through our dedicated innovation group. These sophisticated ex vivo experiments provide additional confidence to clients and their in...
-
Product Physiosources
Physiosources is a french food supplement brand developped & manufactured in Brittany, France. +200 registered formulas addressing various needs such as joint care, immunity, digestion, sleep, ... packaging and label can be adjusted to fit your local market and specific regulatory requirements.
-
Product Glutaredox
GLUTAREDOX is a food supplement based on reduced Glutathione, Vitamin C,L-Cystine and Selenium with patented-release technology.
-
Product KOMKSM-66 Ashwagandha
KOMKSM-66 It is produced using a unique proprietary extraction process, based on “Green Chemistry” principles, without using alcohol or any other chemical solvent. Available many Clinical Studies, Dietary supplement contains 30 capsule in one bottle. Recommended daily dose: One capsule ...
-
Product Redulen® Glicemia
Redulen® Glycemia is an innovative food supplement for blood glucose control based on: • Lemotrin™ a proprietary synergistic complex of flavonoids, • Chromium contributing to the maintenance of normal blood glucose levels* The clinical study shows the improvement in glycemic profile with a sign...
-
Product Tocovid SupraBio
Consist of Mixed Tocotrienols to improve health of Brain, Nerve, and Liver. Tocovid SupraBio helps on reducing risk of stroke & dementia, reduce diabetic lancinating pain and has positive hepatoprotective effect in NAFLD. It's equipped with SupraBio Delivery system to enhance up to 300% absorption of m...
-
Product Solv4U
Poor water solubility of drug candidates is one of the most common hurdles pharmaceutical development is confronted with. Promising new chemical entities and drug candidates often fail in development due to the limitations of their solubility in water.
Marinomed Biotech AG provides Solv4U techno...
-
Product SOMAÍ Inhalation Extracts
SOMAÍ Inhalation Extracts are available in three lines of produtcs in 0.5ml, 1ml for a taylored approach. SOMAÍ Inhalations Extracts Origins and Senses Lines have been enriched with different cultivars and terpenes, allowing them to have different flavors and smells to meet patient'spreferences, our Origin...
-
Product HAWTHORN (CRATAEGUS SPP.) sugar-coated tablets
Support the heart and cardiovascular function.
Dossier in EU-CTD available. Product registered in Germany (Weissdorn-Dragees).
-
Product BLACK GARLIC
Black garlic is really something very special in the traditional healing. It is a kitchen-type garlic (Allium sativum) placed in a special chamber with a precisely defined temperature and humidity for several weeks to stain black in a typical way as a result of fermentation. What is the purpose of that? In...
-
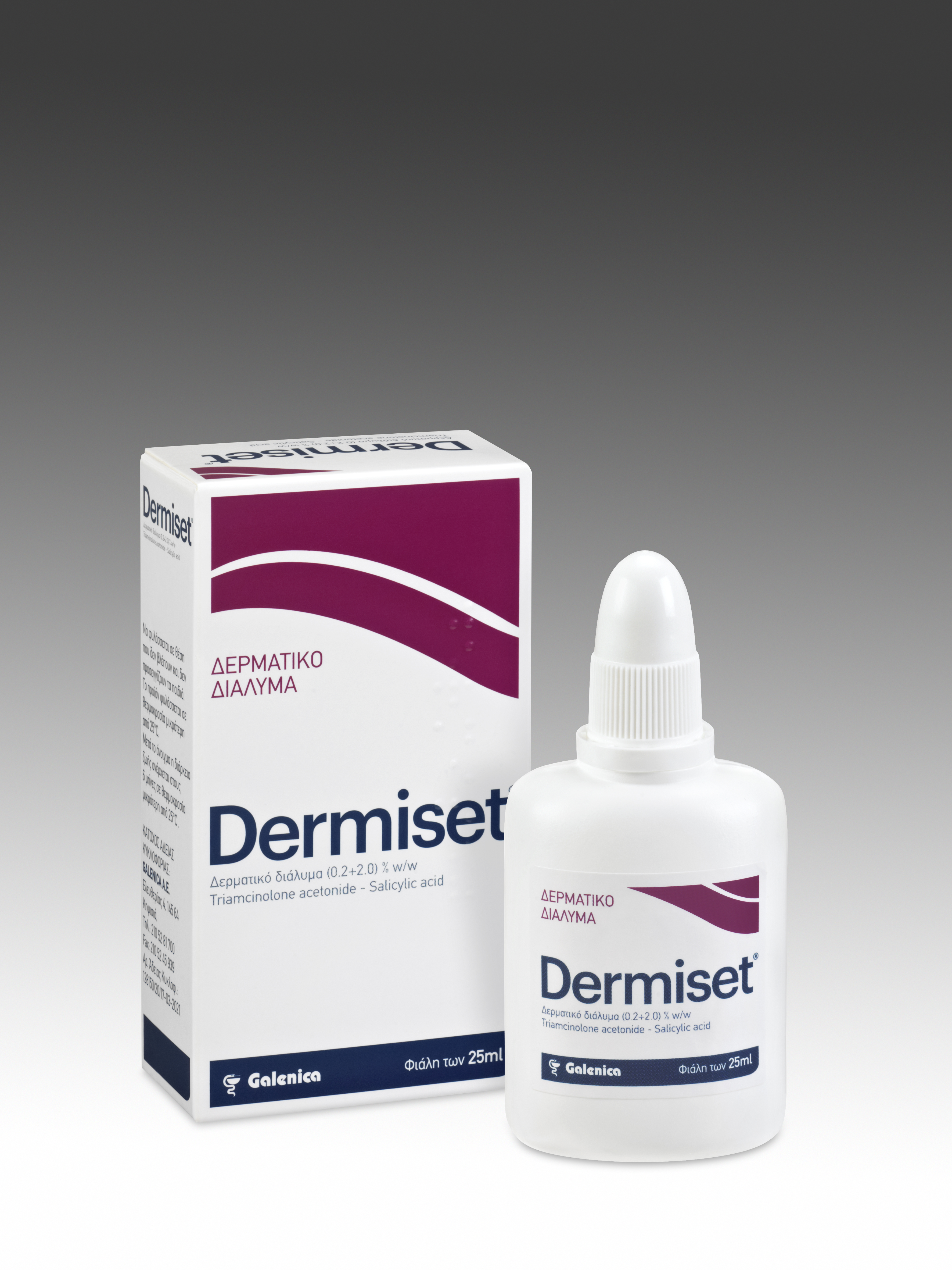
Product DERMISET
Active ingredients:
Triamcinolone Acetonide+Salicylic Acid
Indications:
Eczema and corticosteroid-responsive skin diseases characterized by hyperkeratoses and/or squamous cell formation.
Psoriasis, excluding large psoriasis plaques
Lichenification of the skin.
Neur...
-
Product UniLayer - 2mg Loperamide
This product is suitable for children as a delivery system.
Must be taken with water or beverage of choice.
We are looking to partners on a CDMO bases.

-
Product Iron Sucrose API & Ferrofer(iron sucrose injection)
Iron Sucrose API: CAS No.: 8047-67-4
Ferrofer (Iron Sucrose Injection) is indicated for parenteral treatment of iron deficiency in cases whereby oral iron preparations cannot provide for sufficient supplementation.

-
Product FEBIND-F (deferasirox) 360 MG FILM COATED TABLET
Deferasirox 360 MG FILM COATED TABLET (30 TABS)
FEBİND-F is indicated for the treatment of chronic iron overload (transfusional hemosiderosis) due to blood transfusions in children aged 6 years and above and in adults. This film-coated tablet should not be preferred in the 2-5 age group; instead, ...
-
Product Vitamin Premix Powder
Customized premix solution in powder
We bring you custom nutrient premix blends sourced from high quality ingredients,including vitamins,minerals,amino acids,nucletide,nutraceutical,proteins,sweeteners,prebiotic,fibers,herbs, and more.
-comp286402.jpg)
-
Product MILEDIX®
Miledix ® is a food supplement formulated for well-being during menstrual cycle.
Miledix ® is indicated for the women effected of premenstrual syndrome and dysmenorrhea.
The major clinical symptoms are grief, irritability, depression, abdominal cramps, mood changes, low so...
-
Product Salbutamol Inhalation Aerosol
It is used to prevent and treat respiratory diseases associated with bronchospasm (wheezing) such as bronchial asthma or asthmatic bronchitis.

-
Product Betahistine 12.5 mg/ml Oral Drops
Glass bottle containing 100 ml oral solution with disposal pump.
-
Product 2024- TPA Dosage Forms list
Connecting Taiwan’s pharmaceutical manufacturers with the global marketplace through various business models such as In-Out Licensing, Tech-Transfer, Contract Manufacturing Organization (CMO), and Contract Development and Manufacturing Organization (CDMO). We collaborate closely not only with local manufac...
-
Product Your UTI Free Orosticks
Natural solution for UTI prevention or acute use with an outstanding track record of market response. Supported by 2 clinical studies on the finished product. Launches in various regions are showing exceptional prescription rates and sales due to the product’s efficacy. Based on an exclusive, branded ...
-
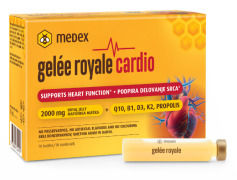
Product Gelee Royale Cardio
Key Ingredients of Gelee Royale Cardio:
• Royal Jelly: The highest content of royal jelly (2000 mg) and HDA (28 mg) so far • Coenzyme Q10: Known for its heart-protective and cardiovascular functions • Vitamins B1, B6, D3, K2, and E: Essential for various aspects of heart and overall health. ...
-
Product Liraglutide injection
for Diabetes and weight loss indication, China marketed drug. CTD form dossier
-
Product OPHTHA HYAL Line - THE EYE, WINDOW TO THE SOUL, WINDOW TO THE WORLD
SafloHyal - in case of OCULAR INFLAMMATION
Key Ingredients: Enoxolone glucuronide 2.5 %, Hyaluronic Acid Sodium Salt 0.10%, PEG/PPG (EG56) 1.5%.
OftaxHyal - in case of CORNEAL INJURIES
Key Ingredients: Hyaluronic Acid, Glyceryl...
-
Product Levofloxacin
Nanjing sino pharmaceutical ltd. Provides wide range of products which includes Levofloxacin 500mg tablets and injections. Indication: Levofloxacin is used to treat bacterial infections in many different parts of the body. Contact us for more information.
-
Product Eslotin (Tablets)
Desloratadine, 2.5 or 5 mg.
Available in 10, 20, or 30 Film-Coated Tablets.
-
Product Paclitaxel for inj (Albumin - Bound) – Paclitaxel White Oak 5 mg/ml powder for dispersion for infusion 100mg/20ml
注射用紫杉醇(白蛋白結合)用於治療對聯合化療無效且已轉移的乳腺癌。
-
Product Night Cream
Brudy Cosmetics Night Cream, with our molecule Algatrium-AL, which is 6 times more powerful than Coenzyme Q-10 and Retinol. Which make us the most powerful antiaging product known.The molecule is patented by us and we only give exclusive distributions.
It is vegan and it goes with a very sophisticated a...
-
Product Innovative Food Supplements
GAP offer its own brand for Food Supplements for Distribution or Pvt LabelOur formulas developed are ALL based on EFSA claims and cover many therapeutical categories: (ex. Mental Health, Sleep Disorders, Boosting Imune System etc)
For full range please refer of the formulas we offer please g...
-
Product Liquid Solutions
Chemical solutions (GMP-compliant): • API & excipients • Hazardous materials • Explosives • Filling under inert gas
Special extracts and tinctures: • Mother tinctures • Boswellia extract • Opium tincture • Capsicum extract • Henbane extract • Cannabis extract
Packaging • 2...
-
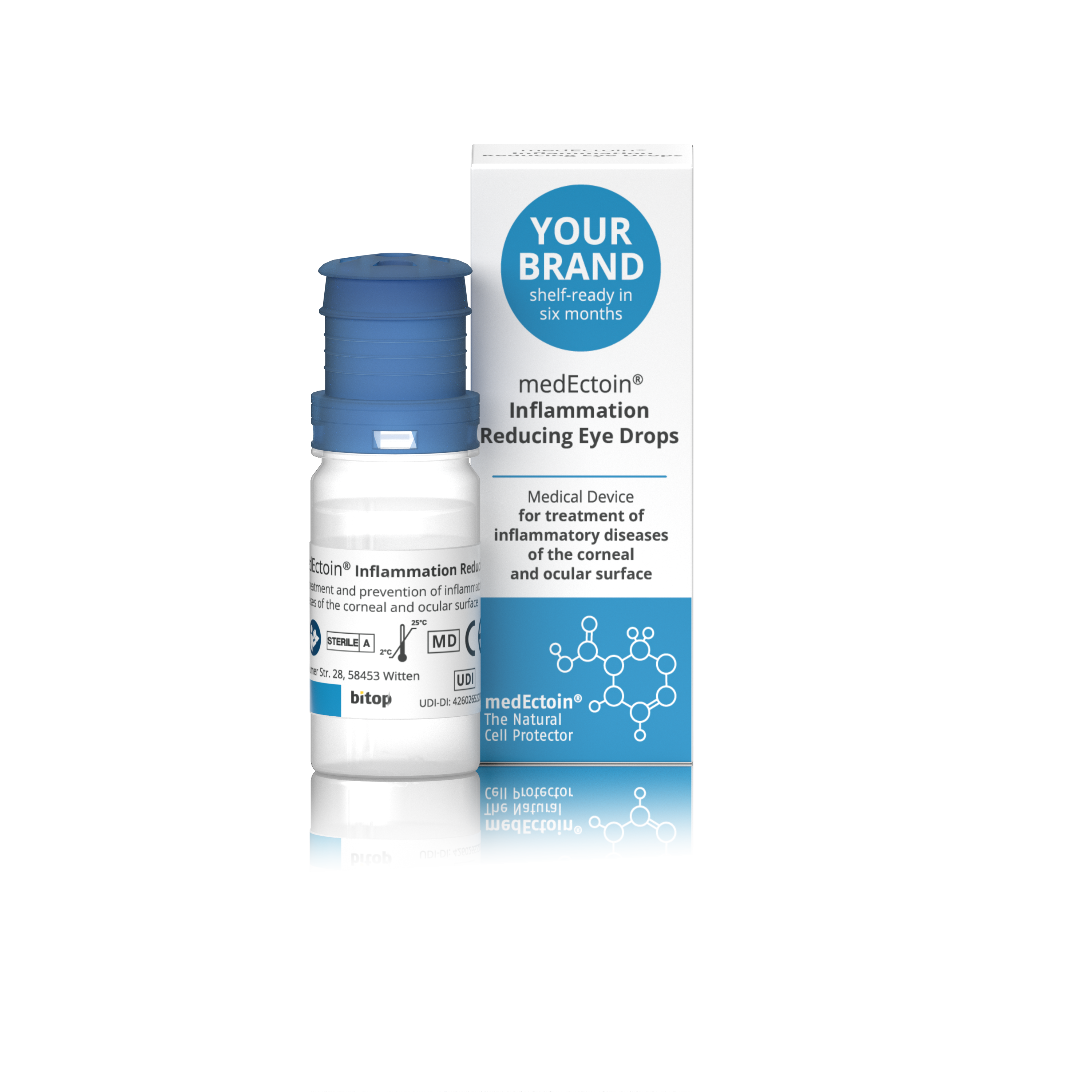
Product medEctoin Inflammation Reducing Eye Drops
Medical device for treatment of inflammatory diseases and the corneal occular surface. • Quick symptoms reduction like itching, tearing and irritation within 30 seconds. • Alleviates symptoms such as burning, dryness, itching, redness, foreign body sensation, etc. • Reduces inflammation...
-
Product Biorela® Choco Multi Kids
Biorela® Choco Multi Kids is the ultimate innovation in children’s food supplement category. This unique children’s multivitamin with premium probiotics in a delicious chocolate form helps in a wide range of everyday challenges: it supports immune and digestive health, kids’ healthy growth and developm...
-
Product DysmenoCalm
It contribute to the subjective feeling of relief, comfort and well-being for those women who suffer a lot of pain during menstruation.
Cedarwood, Butcher's broom, Mint, Lavender, Aloe Vera.
Tube of 50 g
Shelf life: 3 years.
COSMETIC

-
Product Bacitracin vials
Bacitracin is comprised of a polypeptide complex and bacitracin A is the major component in this complex active against a variety of Gram-positive bacteria and a few Gram-negative bacteria, however only staphylococcal infections qualify for the systemic therapy.
Indi...
-
Product Amnion Flush Solution®
Amnion Flush Solution® for continuous amnioinfusion is the first certified treatment option that permanently restores the physiological foetal environment after the preterm premature rupture of membranes (PPROM).Continuous amnioinfusion regenerates the natural amniotic fluid depot via a subcutaneously impl...
-
Product Ginhyal Deli - intimate dryness
Non-hormonal gel based on a patented moisturizing and restructuring compound. Its delicate formulation counteracts vulvo-vaginal dryness and its linked discomfort. It acts as an intimate lubricant and restores intimate mucosa, promoting its natural firmness and elasticity.

-
Product ABIVISOR
ABIVISOR is a food supplement whose composition based on probiotic lactic ferments promotes the balance of the gastro-intestinal flora.
Thanks to its formulation, Abivisor is particularly suitable for subjects in whom altered gastric acidity can cause greater bacterial growth at the gastro-intes...
-
Product Aripiprazole Oral Suspension
Rubicon provides a wide range of central nervous system products which includes aripiprazole ODT. It belongs to Abilify, Otsuka brand. Contact us for more information.
-
Product Bortezomib Onko (Bortezomib), 3.5 mg, Powder for solution for Injection-Vial
Bortezomib Onko (Bortezomib), 3.5 mg, is in Powder for solution for Injection-Vial form. Bortezomib is an antineoplastic agent which is used to treat people with multiple myeloma and mantle cell lymphoma. Contact us for more information.
-
Product Supply Chain Services
With a dedicated team of experienced supply chain specialists, we go far beyond conventional offerings to provide you with innovative and efficient supply chain solutions. With three strategically placed warehouses in Switzerland and Germany, we are able to store and distribute your products in a ...
-
Product SiderAL® Pharmanutra
SiderAL® is the range of supplements with Sucrosomial® Iron, responding to dietary deficiencies and increased iron needs.
Sucrosomial® Technology is used to treat the iron contained in SiderAL® products, making them more resistant to the gastric environment and easier to absorb, avoiding th...
-
Product Baroscon Double action syspension
Relieve acidreflux, heartburn, indigestion, excuess stomach acid

-
Product VITALDOSE® PHARMACEUTICAL GRADE ETHYLENE VINYL ACETATE (EVA)
VitalDose® EVA copolymer is a technology platform for reliable sustained release performance in indications requiring long-acting drug delivery. Common applications of this ethylene vinyl acetate pharmaceutical grade copolymer include subcutaneous and ophthalmic implants and intravaginal inserts. Ther...
-
Product Mirgeal® gel
Mirgeal® gel is an innovative food supplement that rapidly relieves symptoms related to acid reflux performing better than alginate with long-term benefits.
Mirgeal® gel contains licorice extract, documented for its anti-inflammatory and anti-ulcer properties, and Mirtoselect®, a special blueber...
-
Product PANER tablets
Paner is a set of high-quality ingredients, recommended for men, who want to take care of their sexual life quality.
Paner in its composition includes L-arginine, zinc, selenium and valuable extracts of Tribulus terrestris leaves and Siberian ginseng root. Zinc contributes to the maintenance of ...
-
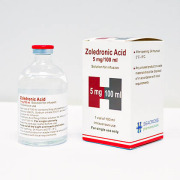
Product Zoledronic Acid Seacross 5mg/100ml solution for infusion
Therapeutic indications:
Treatment of osteoporosis:•in post-menopausal women•in adult men at increased risk of fracture, including those with a recent low-trauma hip fracture.Treatment of osteoporosis associated with long-term systemic glucocorticoid therapy: •in post-menopausal wo...
-
Product Simethicone 80/240/500 mg
Type: Medical device
Pharmaceutical form: Soft-gel Capsules
Package: 30, 15 capsul...
-
Product ALLERGIKA®-Evening Primrose Oil Cream 20% MED
ALLERGIKA®-Evening Primrose Oil Cream 20% MED for the treatment of dry, local eczema!
• The complementary medical device product for dry eczema on hands, arms, legs and body
• The alternative to steroids in mild eczema
• Clinical pro...
-
Product CordenPharma Veterinary Oral Formulation Drug Products
CordenPharma is uniquely positioned to support Veterinary Health companies in the Contract Development and Manufacturing of their Oral Drug Product requirements - with particular expertise and a dedicated, segregated building exclusively for the manufacture of Veterinary Drug Products which have not been p...
-
Product Chorionic Gonadotrophin;Human Choroinic Gonadotrophin;HCG
Source: Extracted from the urine of healthy pregnant womenFunction and use: For women, it can promote the maturation and ovulation of follicles, and maintain the function of the corpus luteum. For men, it can stimulate the development of Leydig cells, increase androgen secretion, promote testicular descent...
-
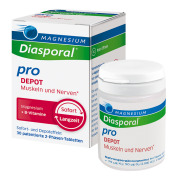
Product Magnesium Diasporal® pro DEPOT muscles and nerves
Magnesium Diasporal® pro DEPOT muscles and nerves is a high-dose combination of magnesium plus vitamin B complex. The patented dual-phase tablet has a fast and slow release: In the short-term phase, immediately active magnesium and the nerve vitamins B2 and B12 are rapidly released. In the long-term phase,...
-
Product Bronchotussine bromhexine cough Syrup OTC
It is used as a mucolytic for the treatment of respiratory disorders associated with productive cough (i.e. a cough characterised by the production of sputum). It has a role as a mucolytic.
-
Product JointCure
Intensive formulas of hudrolyzed collagen, silica and HA, fortified with Mg and many vitamins and minerals, produced in • 30 or20 Oral vials or • Sachets or • Powder 350gr. in Jar
for one month supply
-
Product Drug Products / BD & Licensing
For more than 30 years, Midas is actively providing licenses to market finished pharmaceutical products to its customers in an ever extending international market place. In addition to a range of differentiated finished drug products we offer projects for licensing or acquisition from our proprietary a...
-
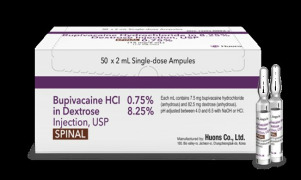
Product 0.75% Bupivacaine HCl in 8.25% Dextrose Injection, USP
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=212822
-
Product Glutathione Injection 600mg
Composition: Each Vial Contains: Glutathione 600mg & One FFS 10ml WFI Brand Name : Glutanc Injection Pack : Combipack
-
Product Charcoal + Enzyme + Probiotics Capsule, Charcoal + Senna + Rhubarb Tablet, Melatonin Sustained Release Capsule, Turmeric Low Dose Capsule, Vitamin C Extender Release Capsule, Caffeine Extended Release Capsule, Taste Masked Fish Omega Oil & Softgels
Nutra / Dietary Supplement / Herbal - Finish Dosage Form
-
Product Diclofenac Sodium Injection
Non-steroidal anti-inflammatory drug (NSAID) used for pain management
-
Product Levohistam
Active ingredient : Levocetrizine Dihydrochloride.
Dosage Form :
Once Daily6 moths – 1 year 5 Drops1 – 5 years 2.5 ml syrup6 – 11 years 5 ml syrupmore than 11 years 1 Tablet
Indication :
For treatment of allergic reactions.
-
Product Panthenol HA Cream 7%
Panthenol is necessary for the preservation of the healthy complexion, beautiful hair and nails. It has been clearly proved that the products containing over 1 % of panthenol have positive effects. New product series Panthenol HA contain also hyaluronic acid. Panthenol and hyaluronic acid are potent binder...
-
Product AQaffron
The only natural adjuvant therapy to antidepressant with proven therapeutic results comparable to pharmaceutical solutions. No side effects. AQaffron is an extremely effective antidepressant aid, demonstrated by our multiple clinical studies on the finished formulation.
-
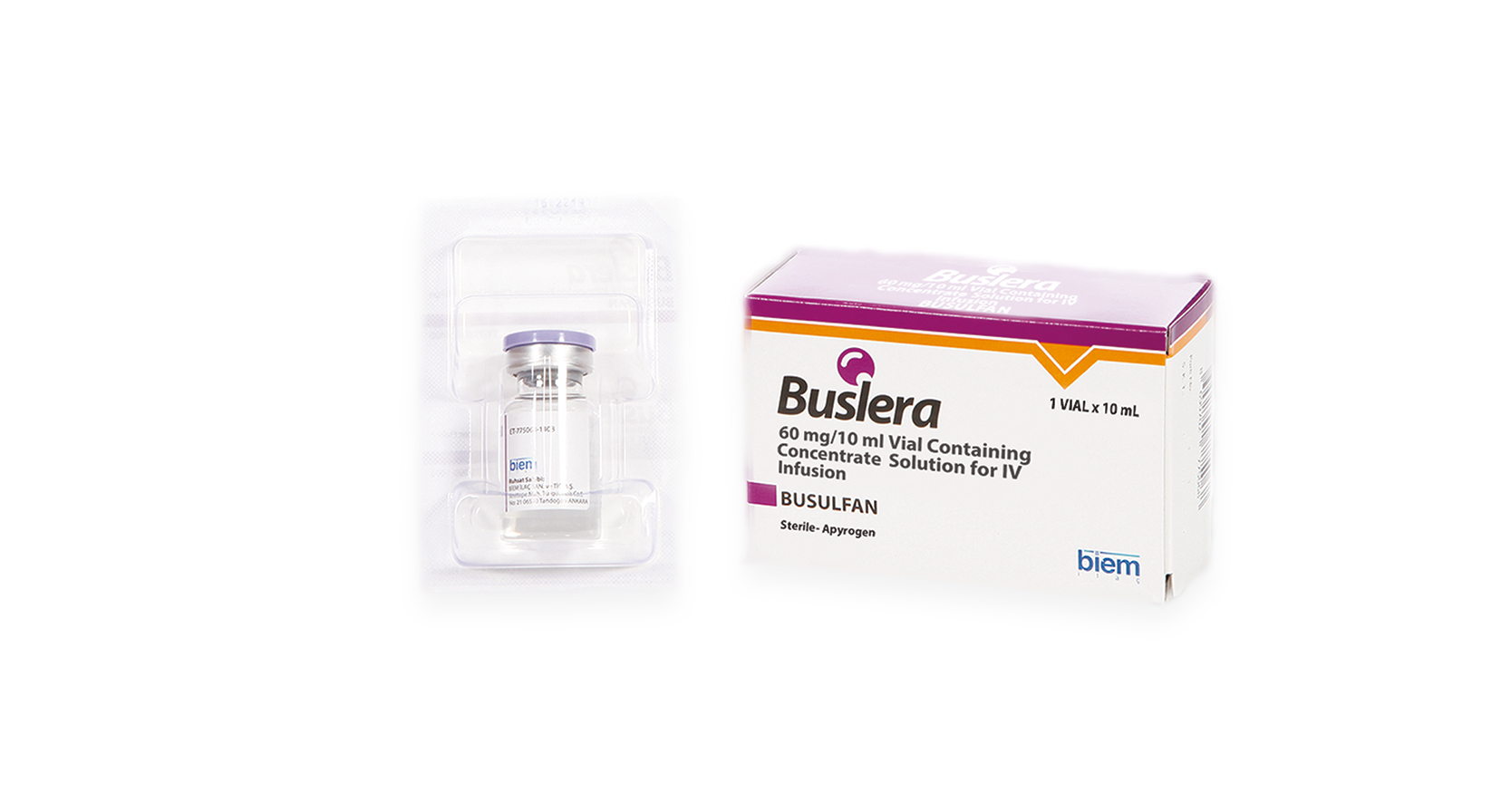
Product Buslera (Busulfan)
BUSLERA 60mg/ 10ml Vial Containing Concentrated Solution For IV Infusion
BUSLERA belongs to “alkylating agents” group of medicines, contains busulfan as active ingredient and used to clear away present bone-morrow before bone-marrow transplations.
-
Product Prosteman
Prosteman product is a unique combination of active ingredients that beneficially affect men’s health, help get rid of unpleasant feelings and release tension caused by the enlarged prostate.Prosteman provides comprehensive care for the prostate. It helps relieve prostate problems and hinders the process o...
-
Product Doxylamine DTM
• Active Ingredient(s): Doxylamine Succinate 12.5/15/25 mg • Unit size: 7/10/14 tablets o sticks per box • Product Description: Useful in the symptomatic treatment of all types of insomnia. Its efficiency is proven from the first night dose. Direct to mouth, no water needed, easy administration, easy to...
-
Product Bifiasthm®
Bifiasthm is a probiotic product proposed for children with asthma, in order to prevent asthmatic crises and support the balance of the immune system reactivitity. Bifiasthm is based on B. breve B632 and L. salivarius LS01, two strains with immunomodulatory activity targeted for allergic asthmatic subj...
-
Product Diclofenac Epolamine Medicated Plaster
MIAT has developed and registered (Hybrid legal basis) in some European countries a medicated plaster based on Diclofenc Epolamine for the symptomatic local treatment of pain and inflammation of rheumatic or traumatic nature of joints, muscles, tendons and ligaments. The product is available only through i...
-
Product CartiJoint Forte
Fidia Farmaceutici Spa offers a wide range of joint healthcare products which includes cartijoint forte. Active ingredient(s): glucosamine hydrochloride (500 mg), chondroitin sulfate (400 mg) and bio-curcumin bcm-95 (50 mg). Indications: dietary supplement containing glucosamine hydrochloride, chondroitin ...
-
Product AROMATIC CITRONELLA PATCHES
Citronella Aromatic mosquitoes Patch. It is an adhesive application with microcapsules on its external side which, when scratched, release Citronella scent. A natural insect repellent with recognized efficacy.• CITRONELLA (Cymbopogon nardus) . Ideal for the Whole family, chi...
-
Product Vitarojal® classic 1000 mg liquid food supplement with royal jelly and honey in vials
Apipharma d.o.o. Offers a wide range of products which includes vitaroja.l 1 vial contains 333.3 mg of lyophilized royaljelly which is the equivalent to 1000 mg offresh royal jelly.Vitarojal® classic 1000 mg contain royaljelly which is known for its biostimulating properties.Each vial contains 1000 mg of r...
-
Product EUDRACAP Functional ready-to-fill capsules
EUDRACAPTM functional ready-to-fill capsules can optimize your release profile, protect your active ingredients, and help accelerate your speed to market. EUDRACAP™ is ideal for use with active ingredients which are sensitive to heat, moisture or gastric acid to optimize absorption and avoid premature ...
-
Product Bioavailability Enhancement - Oral Drug Products and Intermediates
The depth of our bioavailability (BA) enhancement offering makes us a leader in addressing addressing low solubility, low bioavailability and dissolution rate issues. We work collaboratively with our customers to advance compounds, or re-purpose existing compounds, across a full range of API properties and...
-
Product Women's Health
Vaginal Complaints relief, Vaginal Gel Bacterial Vaginosis Infections, Intimate wipes, Menstrual pain heat patch, Intimate (Vaginal) Deo, Menstrual Cup.
-
Product Climavit- Menopause
High quality food supplement based on botanicals - with clinical evidence on support.
The synergy of Genistein, Vitex Agnus Castus and Hawthorn helps hot flushing and nocturnal sweating mitigation as well as night time sleep quality.
Soluble Granules in sachet / ODG in stick
Available for ...
-
Product Gemcitabine
GEMCITABINE 200 & 1000 & 2000 mg parenteral solution – Pancreatic and Breast cancer. Authorized: EU-CTD Dossier
-
Product Nutriregular Macro-Lax
Lazy bowels and constipation. Range of application
• in case of (chronic) constipation
• in case of intestinal irregularities
• in case of Fecalomas
• in case of preparation for diagnostic operations or tests thatrequire the bowel to be empty
Thanks to the osmotic effect o...
-
Product Brimondine Tartrate
Fischer Chemicals AG offers a wide range of dossiers products which includes brimondine tartrate. Category: ophthalmology. Dosage form: eye drops. Contact us for more information.
-
Product Ferrosil plus Vials
Ferrosil Plus is a dietary supplement of Iron, Vitamin C, Vitamin B12 and Folic Acid, useful for filling nutritional deficiencies or the increased needs of these nutrients. Iron contributes to the normal formation of red blood cells and hemoglobin and to normal oxygen transport in the body. Vitamin C incre...
-
Product Basalin®(insulin glargine injection)
Basalin® is an insulin glargine injection developed and produced independently by Gan & Lee Pharmaceuticals. It was launched in China in 2005 and has been approved in almost 20 countries globally. As a long-acting biosimilar insulin, Basalin® is able to stablely control human blood sugar within 24...
-
Product Inhaled Drug Development
Quotient has a 30 year track record in the development and manufacturing of inhaled drug products. We have experience across a range of drug delivery platforms including dry powder inhalers (DPIs) and solutions/suspensions for inhalation. With integrated capabilities and vast knowledge of nasal and pu...
-
Product Products in Private Label
We offer customers a complete range of medical devices for nasal and eye care, ready for customisation with the graphics and brand of the distributor. They are CE-marked and can be sold in EU and non-EU countries, once they have been registered locally.
-
Product Drug Product
Whether you’re developing a new chemical entity or a first-to-market generic, precise dosing and drug delivery is vital for success. Regulatory agencies and clinicians want to see well-characterized, reliable doses with good bioavailability. Patients/consumers often favor a controlled release dosage form, ...
-
Product cGMP Manufacturing Services
Our Operations team has extensive experience in cGMP clinical drug product manufacturing. Our state-of-the-art facilities and cGMP compliant systems are specifically designed for quick-to-clinic operations. Eurofins CDMO’s team of Engineers, Technology Transfer specialists, and Scientists specialize in pro...
-
Product DeCoral Calcium
DeCoral Calcium contains vitamin D3 and bioavailable source of calcium in a digestible form. Coral calcium contains organic calcium 40% and 72 other micro and macro elements that prevent calcium leaching from bones, restores structure of bones and cartilage tissue. Vitamin D3 increases ...
-
Product Ulinastatin for Injection (UTI)
UTI is a standard therapy for physicians and surgeons to treat the patients with Acute Pancreatitis, which can Protect the organ function and Reduce the incidence of complication and mortality.
UTI is a unique treatment choice for physicians to treat the patients with ACF and Sepsis, in E...
-
Product EU GMP Oral Solid Dosage List
LODAAT's Oral Solid Dosages are EU-GMP facility approved and are available in innovations such as ODT, Sachets, and Extended Release. Our facilities handle all testing, production and full packaging capabilities.
-
Product Sevoflurane for Inhalation
Sevoflurane for Inhalation
Product Details
Indication
Sevoflurane is indicated for the induction and maintenance of general anesthesia for in-hospital and outpatient surgery in adult and pediatric patients.
Clinical pharmacology
Sevoflu...
-
Product Spasmoil
Spasmoil is a natural antispasmodic product that consists of two components – peppermint and fennel essential oils for gastrointestinal tract functions. • Peppermint essential oil is the strongest natural component against variety of digestive problems including: Irritable bowel syndrome, spasms, ind...
-
Product Marimer baby Single doses nasal hygiene
MARIMER BABY Nasal hygiene single doses are recommended for babies, children and adults for the daily cleansing of the nasal passages, especially in case of dried or obstructed nose.It can be used daily for preventative purposes, thanks to its physiological concentration (isotonic -9%). A g...
-
Product Doligrippe
API: Paracétamol, Acide ascorbique, Maléate de phéniramine
Dosage form: 500mg + 200mg + 25mg - Box of 8 Sachets
Contact us for more information -
Product PREGNADERM (Pregnancy care products)
Pregnaderm® Extreme Hydration Body CreamHydration - Protection - Enhances elasticityNon-greasy formulation
TUBE of 150 ml
Pregnaderm® Whitening Face Cream SPF 15
Effective control of dark spotsSun Protection - Moisturizing action
...
-
Product Outsourcing of advanced intermediates, active ingredients and formulations
With an extensive range of products and world-wide experience, Summit Pharmaceuticals Europe is able to provide integrated services for the pharmaceuticals industry, from raw materials and APIs up to finished dosage forms.

-
Product Fujietai Skin Disinfection Solution
Liquid/500ml、1LSkin disinfection of injection site and surgery site,disinfection of hand hygiene ,surgical hand antisepsis.
-
Product Ampicillin sodium for injection
Reyoung Pharmaceutical Co. Ltd. is one of the leading manufacturer and distributor of pharmaceutical products.
It has 31 workshops, which can produce ten categories and more than 400 specifications of pharmaceutical products including tablets, capsules, granules, st...
-
Product Vinpocetine-aconitum 5, 10 Mg Hard Capsules or Tablets
Vinpocetine is a time - tested remedy for the treatments of cerebral blood flow. Vinpocetine reduces the brain cell sensitivity to the lack of oxygen and protects them from damage, does not increasing the total blood pressure. It has been observed that vinpocetine has a positive effect on cognitive functio...
-
Product VITALDIN Probiotic gummies
VITALDIN is the specialist confectionery food supplements brand. VITALDIN allows you to supplement your diet in an indulgent and responsible way, because it has developed functional gummies that give you the vitamins you need, with all the scientific rigor and taste of sweets.
We are ma...
-
Product Zoledronic Acid for Injection
It is a bisphosphonate drug given intravenously to treat some bone diseases. It is used to prevent skeletal fractures in patients with cancers such as multiple myeloma and prostate cancer, as well as for treating osteoporosis....
-
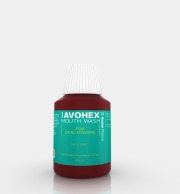
Product AVALON AVOHEX MOUTHWASH 300 ML
AVALON AVOHEX MOUTHWASH is Avalon Recommended product for Oral Hygiene
The Concentration of Chlorhexidine Gluconate 0.2% ideally suited as antiseptic for Oral Hygiene with mint flavor for Fresh and Cool Sensation.
Benefits:- Helps to Protect Teeth and Gum.- Gives cool and Fresh S...
-
Product ICPA PRODUCT BASKET
DERMATOLOGY : Lidocaine+Prilocaine patch & Cream, Mupirocin Ointment, Ketoconzaole Shampoo, Betamethasone cream, Fusidic acid cream, Diaper rash cream and many more..
NASAL & THROAT SPRAY: Mometasone Spray, Budesonide Spray, Fluticasone Spray and many more...
ORAL CARE: Lido...
-
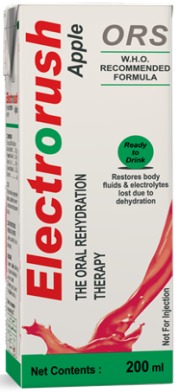
Product Electrorush
Electrorush is a flavored and also with juice , ready-to-drink Oral Re-hydration Solution that provides children and adults with the appropriate amount of electrolytes and fluids they need during mild to moderate dehydration. Concept: Instead of mixing the ORS powder in water, this is...
-
Product SFERAFER
Food supplement useful to fill the food shortages or increased organic iron requirements in case of iron deficiency anemia, abundant menstrual cycle, pregnancy, gastrointestinal pathologies or disorders, vegetarians and vegans diet. Patented with in vitro study. (Bivalent Iron, Copper, Vitamin C). Gluten f...
-
Product ORS - Electrona
Electrona - ORS - Oral Rehydration Solution / Oral Electrolyte.Electrona is available as tetra pack of 200ml easy and practical for use. It is available in two flavors: Orange and Green Apple. Electrona / ORS is new in Lagap portfolio.
-
Product CAPTOPRIL FDF
Medichem S.A. offers a wide range of finished dosage forms for licencing which includes CAPTOPRIL. Indication: Hypertension Pharmaceutical form: Tablets. Strength: 12.5, 25, 50 and 100 mg. Dossier status: Filed ANDA
-
Product Gamma cough&throat lozenges (100 % natural)
Indications • Improve expectoration and relieve the breath. • Eliminate the feeling of sore throat and scratching. • It has bactericidal and anti-inflammatory effect. GAMMA lozenges provide expectorant, anti-inflammatory, analgesic and anti-microbial action, as well as help to reduce...
-
Product Midazolam Injections
Strength: 1mg/ml in 5ml Ampoule : 5mg/ml in 1ml, 3ml and 10ml Ampoule respectively. Contact us more information
-
Product NOVA1947 MICROENEMAS Adults and Children
Laxative containing glycerol. Short term treatment of occasional constipation for adults and children. Form: microenemas Pack size: 6 microenemas Regulatory Class: Medical Device class I
-
Product MANA PHARMA, S.L.
MANA PHARMA, S.L. is a fast-growing Spanish Pharmaceutical Company holding a Pharmaceutical Wholesaler authorisation (WDA). We are specialised in exportation and commercialisation of medicines, medical devices, cosmetics and other pharmaceutical products outside the EU. MANA PHAR...
-
Product Ropivacaine Injection
Ropivacaine Mesylate Injection
Strength:
10ml:90mg;
10ml:120mgbrhrbrA specialist Ropivacaine manufacturer in China
Pls contact me at jiangping@libang.com.cn -
Product Softgels
Softgel Capsules
EnterlCare® Enteric Softgels
Twist-Off Softgels
LiquilSoft™ Chewable Liquid-Filled Softgels
Versatrol™ Controlled-Release Softgels
Solvatrol™ Enhanced Solubility Softgels
Soflet® Gelcaps
Chewels® Chewable Gels
EcoCaps™ Non-Animal Softgels
-
Product Multi-enzyme Digestive Tablets
Digestive disorders has become a common occurrence in today's times due to unhealthy and stressful lifestyle. This hampers overall health and well-being subsequently leading to various related disorders. Multi-enzyme Digestive Tablets consist of essential Enzymes which help in digestion of a broad range of...
-
Product Sachets
Delpharm group offers pharmaceutical services which includes sachets. It belongs to solid forms category. Sachets. Contact us for more information.
-
Product Cefaxone (1 gm i.v)
Pharco corporation offers a wide range of generic drugs which includes cefaxone (1 gm i.v). Contact us for more information.
-
Product LACOSAMIDE
50, 100, 150, 200 mg tablets | Therapeutic use: Treatment of partial-onset seizure | ATC: N03AX18
-
Product Cefaclor particles
Hainan Haiyao Co. Ltd offers a wide range of products which includes cefaclor particles. Packing: 0.125g / bags * 6 bags / box. Formulations: Granule. Function type: Antibiotic. Application: it is mainly applied to the respiratory system caused by susceptible strains of the urinary system, ENT and skin and...
-
Product Aloedep succo immuno
Erbozeta S.P.A offers wide range of cardiology products which includes aloedep succo immuno. Indications: aloedep(R) succo immuno is a food supplement based on herbal extracts. Vita-min c contained in acerola and echinacea supports the normal function of the immune system. Contact us for more information.
-
Product Omeprazole oral solution (10mg/15ml; 20mg/15ml )
First ready to drink omeprazole in liquid form
-
Product Protocatechuic acid
Synonyms: 3,4-Dihydroxybenzoic acid CAS: 99-50-3 Molecular Formula: C7H6O4 Molecular Weight: 154.12 Properties: White to slightly brown needle shaped crystals; soluble in hot water, ethanol, and ether, slightly soluble in water, insoluble in benzene and petroleum ether.
-
Product Colact
Uniao quimica farmaceutica nacional s.a. offers a wide range of products which includes colact. Contact us for more information.
-
Product Reflux
Reflux is a food supplement based on Kiwi extract, Aloe Vera, hyaluronic acid and sodium alginate. Kiwi promotes the body's natural defenses, while Aloe has an emollient and soothing action on the digestive system.

-
Product Bortezomib, Bortezomib for Injection (3.5 mg/vial)
Hainan Shuangcheng Pharmaceuticals Co. manufactures apis and generic finished dosage products which include Bortezomib and Bortezomib for Injection (3.5 mg/vial). Contact us for more information.
-
Product Tadanerfi 20 mg (oral dispersible film)
Tadanerfi 20 mg oral dispersible film for erectile dysfunction
a New Innovative Dosage Form That Is Highly Accepted by The Physician and Patients, Easy & Fast Administration.

Find your finished dosage forms suppliers online: our directory lists nearly 500 suppliers offering 2,500+ products that range from creams and capsules to powders, sprays, suspensions and more.
Finished Dosage Formulation
A Finished Dosage Form is a finished drug product resulting from Finished Dosage Formulation (FDF), the various processes involved in production such as manufacturing, testing, and subsequent approval for consumption and delivery to the public.
Active Pharmaceutical Ingredients (API’s), responsible for the results the user gets after taking the dosage forms, are involved in the FDF along with inactive ingredients (excipients) which serve as a medium for the active ingredients to function.
FDF is a critical stage in the product lifecycle and can only be achieved if the manufacturing facility of a pharmaceutical company or Contract Manufacturing Organization is up to a high-end standard. This is a major reason why some companies are into out-licensing since they don’t have the standard manufacturing facility to produce the dosage forms.
Processes Involved in Finished Dosage Formulation Development
The processes involved here are quite complex. Analysis comes first involving the active pharmaceutical ingredients and excipients. A compatibility study is also done between them.
Next is the formulation development which includes the definition of quantitative and qualitative formulas, formulating the manufacturing method, and establishment of in-process controls.
The next process is the main manufacturing process, which then proceeds to quality control, clinical trials, stability studies and manufacturing regulations before the drug product is transferred to the client if it’s been conducted by a pharmaceutical contract manufacturing organization.
What are the Benefits of Outsourcing Finished Dosage Formulation Development?
Formulation development is an integral process for any commercial manufacturing pharma company and even for private purposes in the development of solid dosage forms. This is why outsourcing always comes in handy because the pharmaceutical contract manufacturing firms are usually more equipped with state-of-the-art manufacturing equipment.
They also ensure that the drug product meets the required industry benchmark using the right method development and standard clinical trial materials to ensure there are no loopholes.
Overall, outsourcing ensures that the dosage form is up to the best standard and is duly regulated.
Growth in Finished Dosage Formulations Market
The key factors that are driving trends in the finished dosage formulation market are:
• Access to cutting-edge technological resources in the global pharmaceutical industry.
• An increased need to outsource drug development and pharmaceutical contract manufacturing.
• The increasing contract manufacturing market share of the industry.
Laws and Regulations for the Manufacture of Finished Dosage Formulation
The US FDA’s Current Good Manufacturing Practice regulations require three successful full-scale batches of finished dosage formulation. These three validation batches are essential to ensure that process design and development studies are up to the required standard for active pharmaceutical ingredients, excipients, and drug production generally. GMP compliance is vital to acquire a license to manufacture finished dosage formulations like soft gelatin capsules.
Finished Dosage Formulation
A Finished Dosage Form is a finished drug product resulting from Finished Dosage Formulation (FDF), the various processes involved in production such as manufacturing, testing, and subsequent approval for consumption and delivery to the public.
Active Pharmaceutical Ingredients (API’s), responsible for the results the user gets after taking the dosage forms, are involved in the FDF along with inactive ingredients (excipients) which serve as a medium for the active ingredients to function.
FDF is a critical stage in the product lifecycle and can only be achieved if the manufacturing facility of a pharmaceutical company or Contract Manufacturing Organization is up to a high-end standard. This is a major reason why some companies are into out-licensing since they don’t have the standard manufacturing facility to produce the dosage forms.
Processes Involved in Finished Dosage Formulation Development
The processes involved here are quite complex. Analysis comes first involving the active pharmaceutical ingredients and excipients. A compatibility study is also done between them.
Next is the formulation development which includes the definition of quantitative and qualitative formulas, formulating the manufacturing method, and establishment of in-process controls.
The next process is the main manufacturing process, which then proceeds to quality control, clinical trials, stability studies and manufacturing regulations before the drug product is transferred to the client if it’s been conducted by a pharmaceutical contract manufacturing organization.
What are the Benefits of Outsourcing Finished Dosage Formulation Development?
Formulation development is an integral process for any commercial manufacturing pharma company and even for private purposes in the development of solid dosage forms. This is why outsourcing always comes in handy because the pharmaceutical contract manufacturing firms are usually more equipped with state-of-the-art manufacturing equipment.
They also ensure that the drug product meets the required industry benchmark using the right method development and standard clinical trial materials to ensure there are no loopholes.
Overall, outsourcing ensures that the dosage form is up to the best standard and is duly regulated.
Growth in Finished Dosage Formulations Market
The key factors that are driving trends in the finished dosage formulation market are:
• Access to cutting-edge technological resources in the global pharmaceutical industry.
• An increased need to outsource drug development and pharmaceutical contract manufacturing.
• The increasing contract manufacturing market share of the industry.
Laws and Regulations for the Manufacture of Finished Dosage Formulation
The US FDA’s Current Good Manufacturing Practice regulations require three successful full-scale batches of finished dosage formulation. These three validation batches are essential to ensure that process design and development studies are up to the required standard for active pharmaceutical ingredients, excipients, and drug production generally. GMP compliance is vital to acquire a license to manufacture finished dosage formulations like soft gelatin capsules.
References
Upcoming Events
-
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025 -
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025 -
CPHI & PMEC China 2025
Shanghai New International Expo Center
24 Jun 2025 - 26 Jun 2025
Pharmaceutical Industry Webinars
-
Webinar An Untapped Market in Gender Inclusivity and Equity
-
19th March 2025
-
3pm GMT /4pm CET
-
-
Webinar Pathway to $10/g Biologics Production: Reshaping the Biomanufacturing Landscape
-
18th February 2025
-
3pm BST /4pm CET
-
-
Webinar Leveraging Real-Time Clinical Data to Deliver Certainty in Solubility Enhancement and Modified Release Development
-
12th December 2024
-
3pm BST/ 4pm CET
-
-
Webinar The CPHI Sustainability Collective: A New Initiative to Support a Sustainable Pharma Value Chain
-
10th December 2024
-
3pm BST /4pm CET
-
-
Webinar Key to Success: A CDMO's Pathway to Biologics Excellence
-
5th November 2024
-
3pm BST/ 4pm CET
-
-
Webinar The Changing Dynamics of Global API Manufacturing
-
17th September 2024
-
3pm BST/ 4pm CET
-
-
Webinar Shaping the Future of Italy’s Pharma Market: Trends and Opportunities
-
4th September 2024
-
4pm CET/ 10am EST
-
-
Webinar Fragment-Based Oligonucleotide and Oligopeptide Synthesis
-
30th Jul 2023
-
4pm CET / 10am EST
-
-
Webinar GMP Rationale for Sterile High-Potency/Toxic Pharmaceuticals
-
18th June 2024
-
4pm CET / 10am EST
-
-
Webinar Unlocking Opportunities in the Growing Pharma Landscape of The Middle East
-
5th June 2024
-
3pm CET / 9am EST
-
-
Webinar Exploring Technological Trends in the Future of Pharmaceutical Manufacturing
-
23rd May 2024
-
4pm CET / 10am EST
-
-
Webinar Achieving Manufacturing Excellence Through Digital Transformation
-
16th April 2024
-
4pm CET / 10am EST
-
-
Webinar Made in Africa: What’s Driving Pharma Manufacturing
-
28th March 2024
-
4pm CET / 10am EST
-
-
Webinar Case Study: Risk Management for Annex 1 Sterile Production EMS
-
28th February 2024
-
4pm CET / 10am EST
-
-
Webinar Innovative Strategies for B2B Pharma Marketeers: Driving Value through Content
-
20th February 2024
-
4pm CET / 10am EST
-
-
Webinar Revolutionizing Pharma: Data and AI Unleashed
-
18th January 2024
-
4pm CET / 10am EST
-
-
Webinar Optimal Temperature: Elevating Biologics Cold Chain Excellence
-
16th January 2024
-
4pm CET / 10am EST
-
-
Webinar Market Outlook – The Biggest Pharma Trends of 2024
-
12th December 2023
-
4pm CET / 10am EST
-
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

















































































-comp241600.jpg)


































































































































.png)





































































%2010.png)























































































.png)



.png)







.png)

.png)



.jpg)
