Directly Compressible Granules
Product Description
YSK Laboratories Pvt Ltd

-
IN
-
2023On CPHI since
-
1 - 24Employees
Company types
Primary activities
Categories
Specifications
YSK Laboratories Pvt Ltd

-
IN
-
2023On CPHI since
-
1 - 24Employees
Company types
Primary activities
More Products from YSK Laboratories Pvt Ltd (3)
-
Product DC Granules Product List
DC Granules Product List -
Product DC Granule Product List
Directly Compressible Granules -
Product Finished Product List
FDF Product List
YSK Laboratories Pvt Ltd resources (9)
-
News YSK Labs - CCD partners Watchlist 2023
YSK Labs was featured on the CCD partners Watchlist 2023 aimed to highlight the profile of Innovative and disruptive Startup companies participating in the CPHI Startup Market 2023. This Watchlist is designed to capture and track exciting up-and-coming businesses with the potential to disrupt the pharmaceutical industry.
Out of approximately 1000+ companies in over 57 countries, YSK Labs was amongst the 21 companies selected and featured. -
Brochure YSK Watchlist 2023 by CCD Partners
We are extremely delighted to announce that YSK Laboratories Pvt Ltd was recently featured in the bespoke Watchlist 2023 by CCD Partners, -
Brochure YSK Laboratories Pvt Ltd Brochure
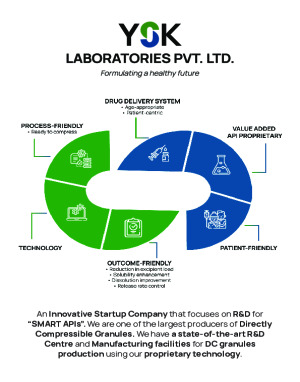
YSK Laboratories is a distinguished R&D company with an integrated manufacturing facility near Mumbai specializing in producing innovative pharmaceutical solutions, particularly our game-changing DC Granules. These Semi-Formulated APIs can be Directly Compressed into Solid Finished Dosage Forms (Tablets) in just ONE compression step. We take pride in offering Superior Quality: SUSTAINED RELEASE/EXTENDED RELEASE (SR/XR/ER) AND IMMEDIATE RELEASE (IR) PRODUCTS which stand out due to their unique Manufacturing Technologies and Lower Excipient Load. Our state-of-the-art production facilities stand as a testament to our unwavering commitment to quality. We maintain a capacity of 400-500 metric tons of DC Granules annually ensuring uninterrupted innovation, production and adherence to the highest industry standards and stringent norms including USFDA, EU GMP, UK MHRA, TGA & WHO GMP demonstrating our dedication to Quality and Regulatory Compliance.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance