Brochure
18 Oct 2024
Mabion - Your Biologics CDMO

PDF 9.0 MB
A description of Mabion's CDMO offering
Content provided by our supplier
Mabion SA

-
PL
-
2023On CPHI since
-
3Certificates
-
250 - 499Employees
Company types
Primary activities
Other Content from Mabion SA (7)
-
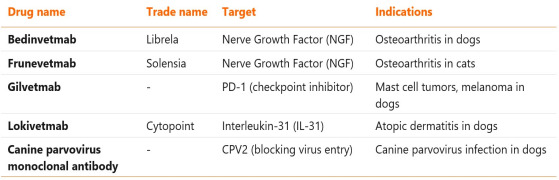
News Development and regulation of veterinary monoclonals
Simarly to the drugs for human use, veterinary drugs must pass a strict registration procedure, both in the EU and US realms – sponsoring companies must present comprehensive evidence that they are safe and effective for use. While in the EU veterinary mAbs are approved by the EMA, in the U.S. these products are evaluated either by FDA or USDA (U.S. Department of Agriculture), depending on the intended indication. -
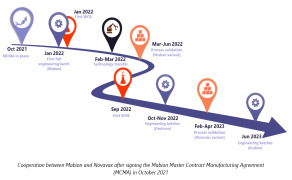
News A synergy of excellence: Novavax-Mabion partnership during unprecedented times amid the COVID-19 pandemic
The COVID-19 pandemic caused an unprecedented crisis that had to be quickly addressed by the development of effective vaccines. Novavax was among several companies that took up the gauntlet and managed to introduce a vaccine (Nuvaxovid) in less than two years since the beginning of the pandemic.
Starting in late 2021, Mabion supported Novavax in their endeavor by performing manufacturing and analytical services. High quality and timely performance enabled the extension of the cooperation to include more QC activities, such as those related to its updated XBB.1.5 strain vaccine. -
Brochure Mabion - Your Biologics CDMO Partner: Drug Substance Manufacturing, Process Development, Analytics, Fill & Finish
With a comprehensive panel of services and fully integrated solutions, Mabion is ready to become your CDMO partner for the entire development journey from pre-clinical to GMP manufacturing. Our end-to-end offer covers every stage of this process, from gene synthesis to the final product release. With Mabion’s expertise and comprehensive services, we can bring your biologic to market rapidly and robustly.
-
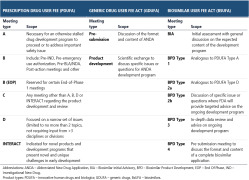
News Similar but not the same: an in-depth look at the differences between EMA and FDA
Pharmaceutical professionals not involved in regulatory affairs may sometimes perceive EMA and FDA as near-mirror images, regulating medicines in different territories yet operating in a similar way. While there are many similarities between the two agencies, several significant differences do exist and can be of paramount value when preparing the drug development strategy.
The contrasts between EMA and FDA are most evident in their legal frameworks, industry consultations, and drug approval pathways. Conversely, regulations concerning clinical research, manufacturing and quality, and post-marketing surveillance are highly similar.
Despite some critical differences, outcomes of EMA and FDA assessments are highly congruent for both biologic and non-biologic medicines. Cooperation between the two organizations is evolving, making the drug registration process more streamlined and less expensive for the pharmaceutical industry. -
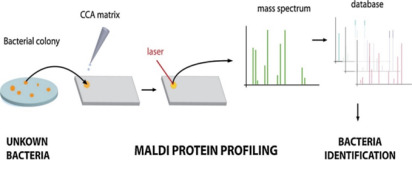
News MALDI-TOF MS techniques in microbial identification
MALDI-TOF MS is a highly sensitive, cost-effective, and fast method for identification of bacterial, viral and fungal species. Its applications continuously expand but the most important one is rapid microorganism detection in pharmaceutical industry.
The technique analyzes ribosomal proteins unique to specific microorganisms by creating a mass spectrum “fingerprint” compared against a database for identification. This allows for the identification of up to 96 isolates in just 1.5 hours.
While highly effective, the technique faces challenges such as smaller library spectra for environmental strains and the need for improved databases and sample preparation methods. Despite these challenges, MALDI-TOF MS remains a promising tool for various microbiological applications due to its speed, precision, and cost-efficiency. -
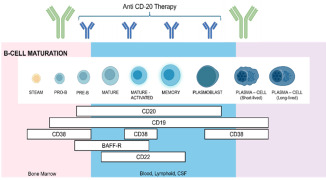
News Bioanalytical assay strategies and considerations for validation of B cells-depletion assay by flow cytometry
Pharmacodynamics of B-cell-depleting therapeutics is assessed with a combination of direct and indirect methods, including flow cytometry, which is considered a gold standard for measuring B-cell counts in whole blood.
Sensitivity is a crucial parameter in bioassays, especially those designed to monitor the pharmacodynamics of cell-depleting therapeutics. Assessing and validating sensitivity involves addressing challenges related to biological variability and lack of biological standards with different levels of dedicated cell populations.
“Fit-for-purpose” validation of a flow cytometry assay ensures that the assay is appropriately validated for its intended use, balancing the level of validation effort with the criticality and intended application of the assay. -
News Mabion signs contract to purchase new bioreactors, diversifies technology, and opens to new customers
On July 11th, 2023, Mabion concluded an agreement with Global Life Sciences Solutions Polands Sp. z o.o., a Cytiva group company for the purchase of a set of Xcellerex XDR bioreactors with conventional mixing technology. The contract is valued at EUR 3.2 million.The vendor will manufacture and install a set of bioreactors in Mabion's facility in Konstantynów Łódzki, Poland, delivery of which is scheduled for Q3 2023, followed by installation, commissioning, qualification testing and acceptance of the equipment. The Xcellerex XDR bioreactor suite is based on technology using a conventional mixing system, as opposed to Mabion's current orbital shaking technology, which will allow the Company to achieve technological diversification, as outlined in the 2023-2027 Strategy in earlier this year.
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance