Similar but not the same: an in-depth look at the differences between EMA and FDA
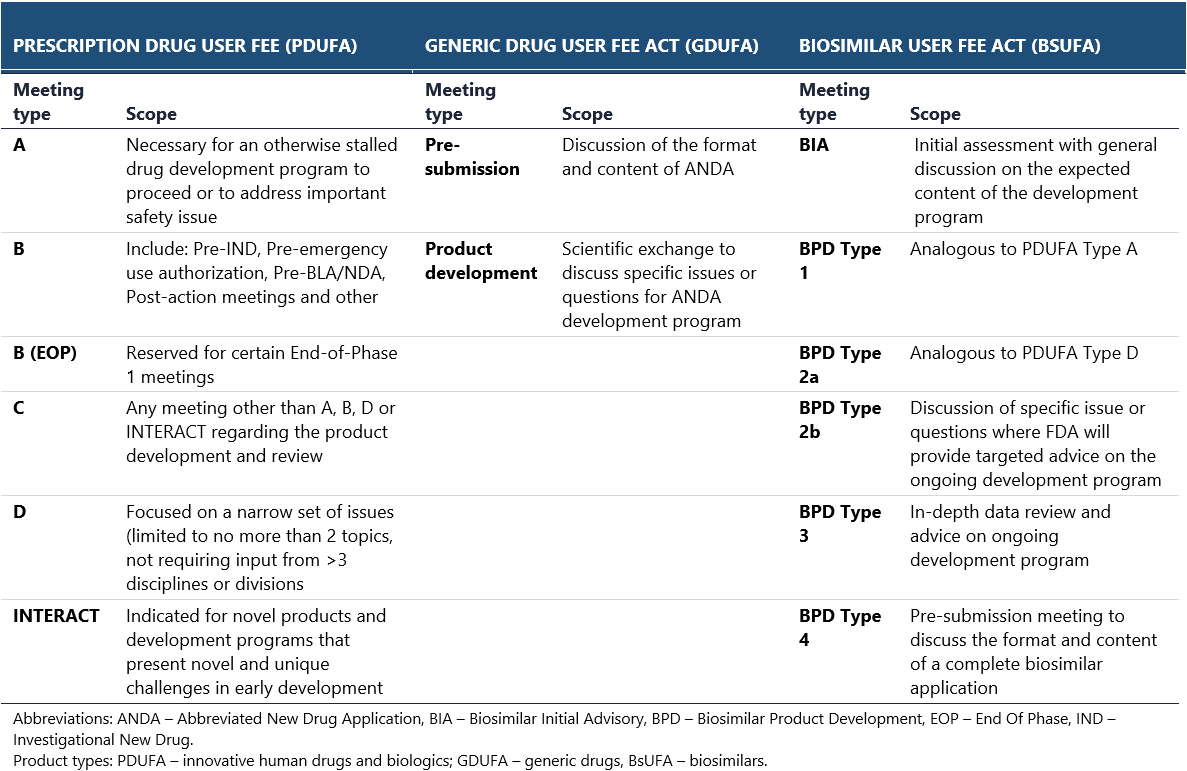
Consultations with the FDA and the EMA
Pharmaceutical professionals not involved in regulatory affairs may sometimes perceive EMA and FDA as near-mirror images, regulating medicines in different territories yet operating in a similar way. While there are many similarities between the two agencies, several significant differences do exist and can be of paramount value when preparing the drug development strategy.
The contrasts between EMA and FDA are most evident in their legal frameworks, industry consultations, and drug approval pathways. Conversely, regulations concerning clinical research, manufacturing and quality, and post-marketing surveillance are highly similar.
Despite some critical differences, outcomes of EMA and FDA assessments are highly congruent for both biologic and non-biologic medicines. Cooperation between the two organizations is evolving, making the drug registration process more streamlined and less expensive for the pharmaceutical industry.
If you are interested in our services regarding the development and regulatory aspects of biologic drugs, please don’t hesitate to contact us. Mabion’s specialists offer individualised approaches tailored to the biologic and clinical characteristics of the developed drug. Our experience with end-to-end development and manufacturing of biologics gives us an overwhelming advantage over other CDMOs as we perfectly understand the client’s perspective.

Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance