Bioanalytical assay strategies and considerations for validation of B cells-depletion assay by flow cytometry
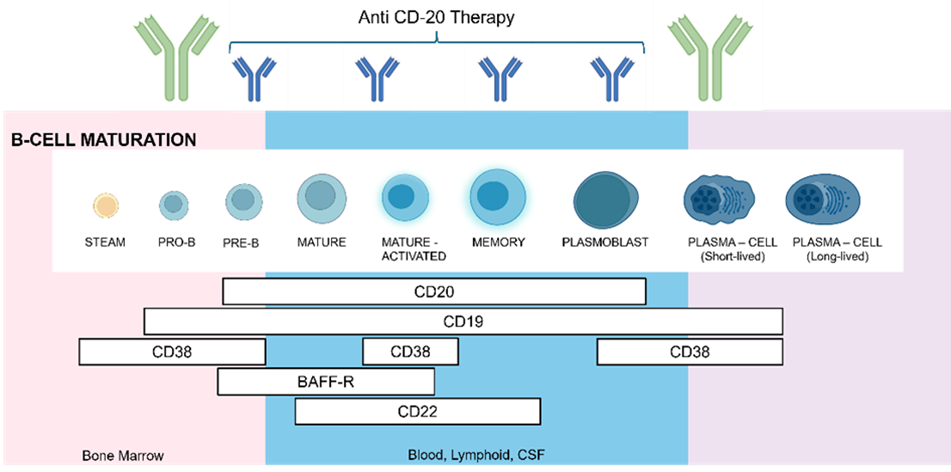
Stages of B cells maturations and expression of cell-surface antigens
Pharmacodynamics of B-cell-depleting therapeutics is assessed with a combination of direct and indirect methods, including flow cytometry, which is considered a gold standard for measuring B-cell counts in whole blood.
Sensitivity is a crucial parameter in bioassays, especially those designed to monitor the pharmacodynamics of cell-depleting therapeutics. Assessing and validating sensitivity involves addressing challenges related to biological variability and lack of biological standards with different levels of dedicated cell populations.
“Fit-for-purpose” validation of a flow cytometry assay ensures that the assay is appropriately validated for its intended use, balancing the level of validation effort with the criticality and intended application of the assay.
Mabion provides comprehensive flow cytometry services tailored to the needs of biopharmaceutical companies, clinical laboratories and research institutions. Our experienced scientists offer robust support for various flow cytometry endpoints using various cell types, including primary cells and human or animal-origin cell lines. Whether you require non-regulated or regulated assays (GMP/GLP/GCP), we can provide phase-apprpriate flow cytometric bioanalysis and confidently assist you utilizing the following platforms: BD FACS Lyric and BD FACS Verse powered by the BD FACSuite Acquisition and Analysis Application.

Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance