Development and regulation of veterinary monoclonals
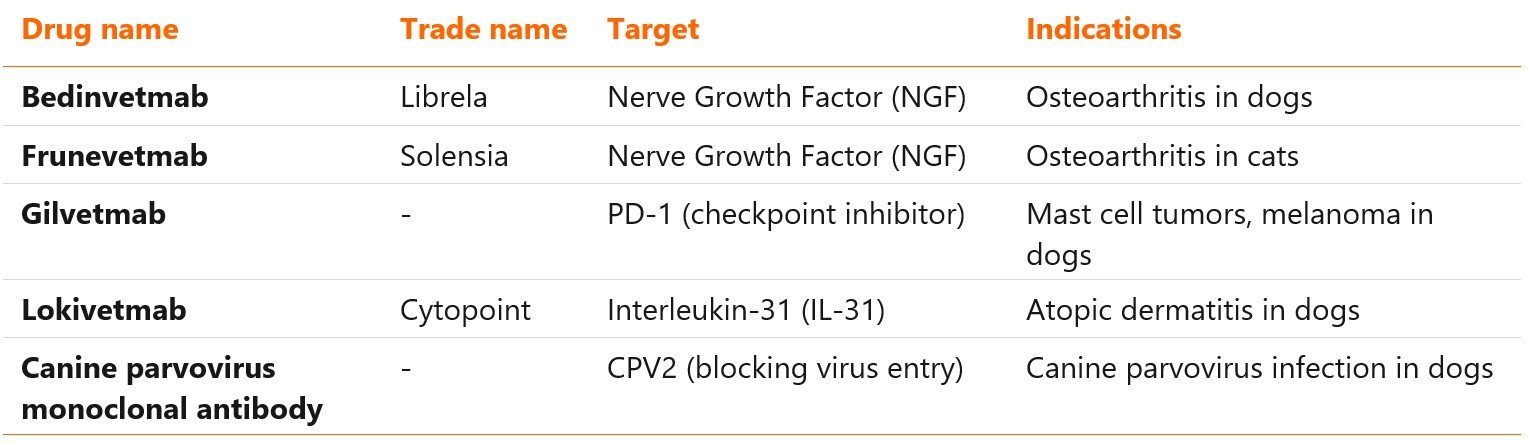
Approved veterinary mAbs
Simarly to the drugs for human use, veterinary drugs must pass a strict registration procedure, both in the EU and US realms – sponsoring companies must present comprehensive evidence that they are safe and effective for use. While in the EU veterinary mAbs are approved by the EMA, in the U.S. these products are evaluated either by FDA or USDA (U.S. Department of Agriculture), depending on the intended indication.
Veterinary monoclonal antibodies are a relatively new class of therapeutics, lagging 30 years behind the human mAbs. Only five molecules have been approved so far: bedinvetmab, frunevetmab, lokivetmab, gilvetmab, and canine parvovirus monoclonal antibody (the last two with conditional approval).
Clinical development of veterinary mAbs is more streamlined that for human monoclonals. Typical pathways include laboratory studies, which confirm the effectiveness on appropriate animal model, followed by field studies with client-owned animals.
Many innovative mAbs are currently being investigated and pharmaceutical companies count the time until first veterinary biosimilars can be introduced to market. However, many years must pass before the patents for veterinary products expire and so far no specific regulations have been issued by drug agencies.

Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance