ZHEJIANG LANGHUA PHARMACEUTICAL CO., LTD
About ZHEJIANG LANGHUA PHARMACEUTICAL CO., LTD
Certifications
Categories

-
CN
-
2015On CPHI since
-
3Certificates
-
500 - 999Employees
Company types
Primary activities
Products from ZHEJIANG LANGHUA PHARMACEUTICAL CO., LTD (44)
-
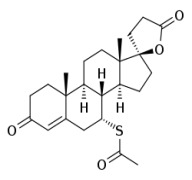
Product Spironolactone
cardiovascular system drugs CEP and USDMF registered -
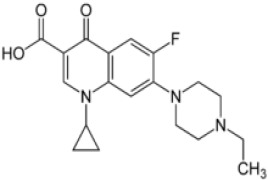
Product Enrofloxacin
Third generation Quinolones antibacterial products, veterinary use CEP and USDMF registered -
Product Levofloxacin Hemihydrate
Quinolones antibacterial product, anti-infective CEP , USDMF registered -
Product Ciprofloxacin Hydrochloride
Wide-range antibacterial -
Product Canrenone
Intermediate grade: Assay: NLT 90%; NLT 97%.
API grade -
Product Olanzapine
USP40/EP9/CP2015DMF -
Product Apalutamide
Apalutamide -
Product Apremilast
Apremilast -
Product Dolutegravir Sodium
Treatment of HIV infection -
Product Eluxadoline
Eluxadoline -
Product Eltrombopag
Treat for thrombocytopenia -
Product Empagliflozin
Empagliflozin
ZHEJIANG LANGHUA PHARMACEUTICAL CO., LTD Resources (10)
-
News CPHI Discover: Two-Tier Contract Market in China as Top CDMOs Grab all the Growth
Ahead of CPHI Discover (17-28 May, 2021) - global pharma's largest ever virtual gathering - we spoke to CPHI Annual Report expert Vicky Xia, China Head at BioPlan Associates on the biologics trends she is seeing emerging in the contract services space in China. -
Video Applications of Green Chemistry in API Production
This webinar originally aired as part of CPHI Discover - 17-28 May 2021 Green Chemistry Overview Introduction to Green Chemistry- Twelve Principles and Examples Green Chemistry via Continuous Flow Advantages and Disadvantages Continuous flow reactors Continuous flow chemistry at Langhua -
Brochure Think of CDMO, Think of Langhua
It has passed FDA, WHO, EDQM, ANVISA and CFDA’s inspections, has the ability to ensure the risk-free GMP compliance to support customer NDA filing. Moreover, Langhua excellent EHS system passed PSCI and big pharma’s inspection as well.
Plenty CDMO cases have been completed in Langhua site. Langhua has been engaged in the whole lifecycle of chemical entities, including ROS screening, process development & optimization, scale-up, registration & validation campaign through to commercial supply. With the concept of “Develop for Production” and “Lab to Plant by the same team”, Langhua ensures the timely project transfer from lab to industry with 100% success rate and eco-friendly.
Langhua respects customers’ intellectual property, set up the procedure to protect customer’s confidential information. Together with subsidiary company Nuobai PharmPharmaceutical Co., Ltd. Langhua can provide customer one-stop solution, including Custom Synthesis, Global Mark...
-
Brochure Nuobai Pharm-Pharm Intermediates Division
At Nuobai, with over 30 years experience and operating in Europe, North and South America, India, Japan and Korea, Nuobai Pharm offers an extended range of services from sourcing, consultancy to manufacturing with a special focus on R&D, intermediates. Nuobai Pharm acts as an extension to your team by providing expert advice and counsel. Nuobai Pharm supports in technical docmentation, pre-shipment test, and site audit etc, to improve supply quality, reliability and reduce supply chain risk. From initial research and business development to commercial manufacturing and life cycle management, Nuobai Pharm takes your project at any stage and moves it forward by selecting the most suitable development and manufacturing partner. It is our mission to be your reliable partner offering high efficiency for your requirements in today’s dynamic business environment. -
Brochure Nuobai Pharm-Formulation Division
Caring for life health, Nuobai Pharm has been devoted to supplying safe, quality and cost-effective pharmaceutical products. In 2015,2016,2017 Nuobai Pharm was awarded top 10 exporters of pharmaceutical formulations by Chinese government. Nuobai Pharm has a wide range of products, covering human medicines, veterinary products and medical devices. Products have been registered and marketed in more than 50 countries, which enjoy high reputation in Asia, Africa and Latin America. 80% of our team members have pharmaceutical background and always supply professional one-stop service including business development, registration, GMP audit, order processing, logistic and quality control. More than 50 items have complete CTD format dossiers. All the production workshops have passed the national GMP certification, and the production process is in strict accordance with the GMP requirements. Nuobai Pharm can supply various dosage forms of products for your choice including tablet, capsu... -
Brochure Nuobai Pharm- APIs Division
For APIs, we have clear picture in the market and good relationship with most of API manufacturers all around China. We established our own warehouse and distribute APIs at lower rate. All these activities are performed in line with the latest guidelines and applicable cGMP requirements. -
Video Market Outlook: China
An in-depth look at the Chinese Pharma market. Hear from Associations and Analysts on market potential, challenges and opportunities, and learn from industry case studies on how to build a business in one of the world’s biggest Pharma markets. Why choose to manufacture in China? Overview of Chinese manufacturing capabilities Addressing supply chain and production challenges in light of COVID19 Why manufacturing in China is still a safe, reliable and quality choice Chinese capabilities across the large and small molecule space -
Brochure COVID-19 related products
Anti-coronavirus products processed by subsidary company Nuobai Pharm. Ningbo Nuobai can supply various anti-coronavirus products to protect human health covering Masks, Protective Clothing,Goggles,Gloves, Shield, Pulse Oximeter,Alcohol Pad,Infared Thermometer, Disposable virus sampling kit, Coronavirus disease 2019 antibody(IgG IgM) combined test kit and so on.
-
Brochure Langhua: Your Reliable and Preferred CDMO Partner in China!
With rich information in the file, Langhua together Nuobai's business mode will be easily understood.
In the CDMO business senario, Langhua presents good choices for your new entities and APIs.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





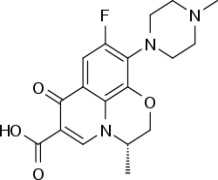
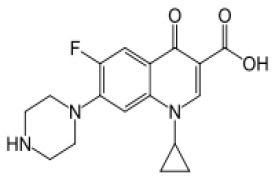
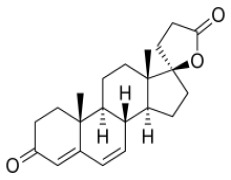
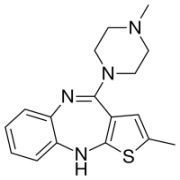
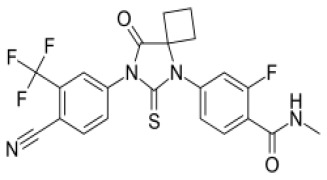
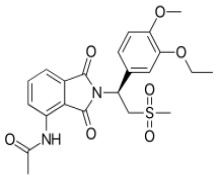
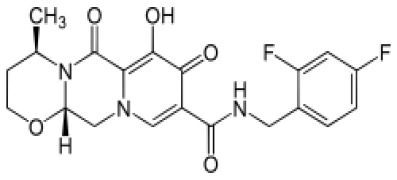

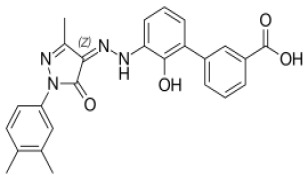







![(3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-ol](https://www.cphi-online.com/46/product/125/79/26/p0th_S.jpg)