Research & Development News
Research & Development news
-
News SIR-Spheres Y-90 resin microspheres are a well-tolerated alternative to standard therapies for inoperable primary liver cancer, says new UK NICE MIB
Unlike TACE, most patients treated with SIR-Spheres Y-90 resin microspheres usually require only a single treatment. -
News SGS and DiscoverX to collaborate on the qualification and supply of bioassays for biopharmaceutical development
The availability of commercial ready-to-assay cryopreserved cells in a qualified kit will remove the need for developers to employ challenging and time-consuming method development procedures. -
News BMS and Pfizer announce global real-world data program and present new analyses of Eliquis at the American College of Cardiology’s 65th Annual Scientific Session
Seventeen abstracts to be presented, including new analyses from the Phase III ARISTOTLE and AMPLIFY clinical studies and from real-world databases. -
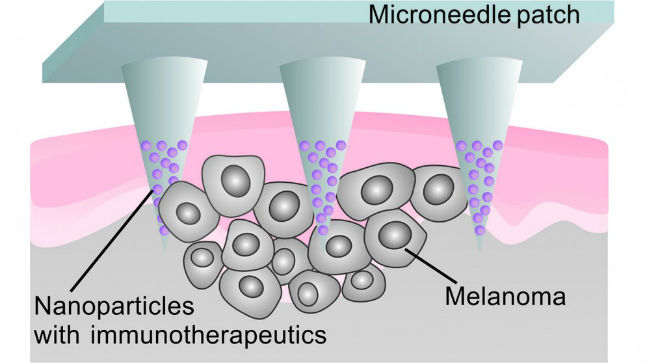
News Microneedle patch delivers localized cancer immunotherapy to melanoma
Researchers developed a patch that uses microneedles to deliver anti-PD-1 antibodies locally to the skin tumour. -
News EMA accepts for review Pfizer's MAA for Xeljanz for the treatment of moderate to severe rheumatoid arthritis
Application provides additional information to the original MAA submission, including data from the Phase III ORAL global development program in RA. -
News Regeneron and Bayer to jointly develop novel combination therapy for eye diseases
Will jointly develop a combination therapy of the angiopoietin2 (Ang2) antibody nesvacumab and the vascular endothelial growth factor (VEGF) trap aflibercept, for the treatment of serious eye diseases. -
News AstraZeneca reports top-line results from the Brilinta SOCRATES trial in stroke
Missed primary efficacy endpoint; fewer events observed in the Brilinta arm but trend did not reach statistical significance. -
News Eisai withdraws NDA for mecobalamin ultra-high dose preparation as treatment for ALS
The application package submitted was not sufficient for approval. -
News Placon Therapeutics announces company launch and FDA acceptance of IND for novel platinum candidate BTP-114
Placon, spun out from Blend Therapeutics, to pursue development of next generation cytotoxic oncology drugs. -
News Lilly's Taltz receives FDA approval for the treatment of moderate-to-severe plaque psoriasis
In pivotal studies, most patients treated with Taltz achieved significant skin clearance with many achieving virtually clear or completely clear skin at week 12. -
News Eagle Pharmaceuticals receives Complete Response Letter from FDA on Kangio application
FDA requests further characterization of bivalirudin-related substances in the drug product. -
News GSK and Miltenyi Biotec establish cell and gene therapy collaboration
Collaboration includes discovery programme for CAR-T cell-based oncology therapies. -
News BMS to present new overall survival data for Opdivo as monotherapy and in combination with Yervoy at AACR 2016 Annual Meeting
New, pivotal Phase III overall survival data from CheckMate -141 for Opdivo in previously treated advanced squamous cell carcinoma of the head and neck, to be presented. -
News RedHill Biopharma announces positive FDA meeting on RHB-105 path to approval and planned confirmatory Phase III study for H. pylori infection
The FDA has confirmed, subject to final minutes of the meeting, the planned two-arm, randomized, double-blind, active comparator design of the confirmatory Phase III study with RHB-105 for the treatment of H. pylori infection, expected to be initiated ... -
News Experimental dengue vaccine protects all recipients in virus challenge study
Vaccine developed by NIH and FDA scientists. -
News Monoclonal antibodies headline innovation in gastric cancer treatment pipeline
GBI Research says pipeline consists of 29% monoclonal antibodies. -
News FDA grants Genentech’s cancer immunotherapy atezolizumab Priority Review for advanced bladder cancer
FDA will make a decision on approval by 12 September 2016. -
News FDA approves Xalkori for the treatment of patients with ROS1-positive metastatic NSCLC
Xalkori is the first and only FDA-approved biomarker-driven therapy for ROS1-positive metastatic NSCLC. -
News PhoreMost and The Wistar Institute collaborate to identify new druggable targets in cancer, immunotherapy and ageing
Collaboration will use Siteseeker — a new ‘phenotypic’ screening platform that exploits the dynamics of a live-cell environment to uncover hidden druggable target sites across the entire human genome — to find new therapeutic options. -
News EMA launches PRIME — paving the way for promising medicines for patients
New scheme supports European Commission priorities. -
News New understanding of the mechanism of neurodegeneration leads to a novel approach to treatment for Alzheimer’s disease
Innovative drug prototype shown to block damaging activity in human cell lines suggests a promising strategy for future therapeutics. -
News FDA accepts sBLA for Keytruda (pembrolizumab) in advanced NSCLC
Application based on data from KEYNOTE-010, which showed superior overall survival for patients taking Keytruda compared to chemotherapy in patients with PD-L1 expression on one percent or more of the cancer cells. -
News Adding Spiriva Respimat effective for uncontrolled asthma, regardless of allergy subtype
Spiriva Respimat shown effective in the broad range of asthma patients studied who continued to experience symptoms despite taking other asthma maintenance therapies. -
News Imbruvica (ibrutinib) approved by FDA for the first-line treatment of chronic lymphocytic leukemia
First FDA-approved chemotherapy-free treatment option for first-line CLL patients. -
News DSMB recommends Celldex's Phase III study of Rintega (rindopepimut) in newly diagnosed glioblastoma be discontinued
Study unlikely to meet primary overall survival endpoint in patients with minimal residual disease. -
News Victoza significantly reduces the risk of major adverse cardiovascular events in the LEADER trial
The safety profile of Victoza in LEADER was generally consistent with previous liraglutide clinical studies. -
News Ixekizumab demonstrates rapid, improvements as among patients with moderate-to-severe plaque psoriasis
In a combined analysis of UNCOVER-2 and UNCOVER-3, significant improvement of psoriasis plaques was observed in patients treated with ixekizumab at 1, 2 and 4 weeks. -
News Onconova Therapeutics receives notice of termination for convenience from Baxalta
Rights to commercialize rigosertib in Europe to revert to Onconova. -
News FDA approves new indication for Faslodex (fulvestrant)
Approval expands use and offers additional option for women with HR+, HER2- metastatic breast cancer. -
News Breast cancer is only pharmaceutical pipeline with over 1000 drugs in development
GBI Research says the pipeline may significantly alter the clinical and commercial landscape of the disease market over the next decade. -
News Merck initiates Phase III study of MSB11022, a proposed biosimilar of adalimumab, in chronic plaque psoriasis
Study will evaluate efficacy, safety and immunogenicity of adalimumab biosimilar candidate MSB11022 compared with Humira. -
News Concert Pharmaceuticals announces CTP-656 solid dose Phase I results confirmed superior pharmacokinetic profile to Kalydeco
Concert is using this tablet formulation of CTP-656 in its ongoing multiple ascending dose Phase I clinical trial and intends to use the same formulation in a Phase II clinical trial. -
News Sobi and Biogen receive positive opinion from CHMP for Alprolix (rFIXFc) for the treatment of hemophilia B
If approved, Alprolix would be among the first therapies in the European Union (EU) to offer people living with hemophilia B prolonged protection against bleeding episodes with prophylactic dosing intervals. -
News Acticor Biotech selects Catalent GPEx Cell Line Development Technology to develop ACT-017
Acticor Biotech is developing an antibody fragment (Fab) considered as a first-in-class anti-thrombotic agent without bleeding risk in the primary treatment of ischemic strokes. -
News Company pioneering nanotechnology to bring innovative medicines that make a real difference
Blueberry Therapeutics is set to create safe, effective “nanomedicines” to treat a number of unmet clinical needs. -
News Chem-Space launches to provide largest global chemical database and search tool
Chem-space.com online searchable marketplace features over 15 million compounds. -
News Eylea outperforms Avastin for diabetic macular edema with moderate or worse vision loss
NIH-funded clinical trial shows Eylea, Avastin, and Lucentis perform similarly when vision loss is mild. -
News True North Therapeutics receives Orphan Drug Designation in the EU for TNT009 for the treatment of autoimmune hemolytic anemia, including CAD
Top-line Phase Ib data in patients with CAD expected in mid-2016. -
News FDA approves new indication for Novartis drug Afinitor for progressive, nonfunctional GI and lung neuroendocrine tumours
In advanced progressive, nonfunctional NET, Afinitor is the first approved treatment for patients with lung NET and the first oral therapy for GI NET. -
News Parkinson’s disease market set to hit $3.2 billion by 2021 as search for cure continues
GBI Research report says that despite steady market growth, various unmet needs remain and a cure is yet to be found. -
News FDA approves Genentech’s Gazyva for certain people with previously treated follicular lymphoma
This is the second FDA approval for Gazyva based on a positive Phase III study. -
News FDA accepts and grants Priority Review for Avycaz sNDA
Application seeks to expand Avycaz label to include Phase III clinical data for the treatment of complicated intra-abdominal infections , in combination with metronidazole. -
News Cobra Biologics and Centre for Process Innovation collaborate to advance development of regenerative medicines
Cobra and CPI will develop in depth scientific and technical understanding to allow a scalable and flexible manufacturing process to be developed to produce, purify and characterise a range of AAV vectors. -
News Novo Nordisk successfully completes fifth Phase IIIa trial with semaglutide in people with type 2 diabetes
The trial successfully achieved its objective. -
News Merck's investigational once-daily formulation of Isentress meets primary and secondary endpoints in pivotal Phase III study
Results to be presented at future medical meeting, and regulatory submissions planned for 2016 -
News FDA accepts Sanofi NDA for once-daily fixed-ratio combination of insulin glargine and lixisenatide
FDA decision anticipated in August 2016. -
News Vaginal ring provides partial protection from HIV in large multinational trial
NIH-funded study finds protective effect strongest in women over age 25. -
News Amgen and UCB announce positive top-line results from Phase III study of romosozumab
Results from the FRAME study showed that women receiving subcutaneous injection of romosozumab monthly experienced a 73% reduction in the relative risk of a vertebral fracture through 12 months compared to those receiving placebo. -
News Mylan's ANDA for generic Advair Diskus accepted for filing by FDA
FDA provides GDUFA goal date of 28 March 28 2017 -
News Aptuit and Icagen agree to strategic alliance
Alliance to provide drug discovery customers access to industry leading ion channel and transporter technologies. -
News Pfizer receives expanded FDA approval for Ibrance in HR+, HER2- metastatic breast cancer
First-in-class therapy now approved for use in a broader range of women. -
News Novartis drug PKC412 receives Breakthrough Therapy designation from FDA for newly-diagnosed FLT3-mutated AML
PKC412 (midostaurin) significantly improved overall survival of adult patients eligible to receive standard induction and consolidation chemotherapy. -
News Impax receives FDA approval for generic version of Adderall XR capsules, CII
Company due to supply its ANDA product during the second quarter of 2016. -
News Merck receives Complete Response Letter from FDA for Zetia (ezetimibe) and Vytorin (ezetimibe and simvastatin)
The company is reviewing the letter and will determine next steps. -
News Incyte decides to discontinue JANUS studies of ruxolitinib plus capecitabine
The decision to stop the study was made after a planned interim analysis of JANUS 1 demonstrated that ruxolitinib plus capecitabine did not show a sufficient level of efficacy to warrant continuation. -
News JHL Biotech receives approval From European authorities to begin biosimilar clinical trial
First biotech company in Greater China region to receive European approval. -
News Newly published head-to-head data show Stiolto Respimat improved lung function across range of measures
Stiolto Respimat was compared with a European formulation of a combination of a long-acting beta agonist, salmeterol, and an inhaled corticosteroid, fluticasone propionate. -
News Synlogic announces first industry collaboration with AbbVie to develop a new class of medicines designed to power the microbiome
Multi-year R&D collaboration with AbbVie focused on novel medicines for treatment of inflammatory bowel disease. -
News Biogen joins pioneering Target Validation Collaboration
The Centre for Therapeutic Target Validation welcomes new member Biogen, expanding its efforts to accelerate drug discovery research. -
News Optibrium supports global health through academic and not-for-profit consortium
Access to StarDrop will support development of new treatments for neglected diseases via global health programme. -
News FDA approves expanded use of BMS’s Daklinza
Updated label provides new treatment option for patients with HIV-1 coinfection, advanced cirrhosis, and post-liver transplant HCV recurrence. -
News Microbix announces new product development program to help combat Zika virus
Using its established development platform and unique expertise in virology, Microbix intends to develop and launch a range of products to help combat the Zika virus. -
News Oasmia submits MAA to the EMA for its lead cancer product Apealea
The indication sought for Apealea is treatment of epithelial ovarian cancer in combination with carboplatin. -
News Affinivax secures additional funding from Gates Foundation to advance its novel pneumococcal vaccine towards clinical trials
Company achieves key milestones and additional $2.5 million from the Gates Foundation. -
News Vertex receives Complete Response Letter from FDA regarding Kalydeco
Company plans to meet with FDA to determine appropriate path forward. -
News Selvita opens new research site in Poznan
The initial laboratory space will amount to 5,500 sq. ft, which constitutes nearly 20% increase in Selvita laboratory space, compared to the current state with significant extension possibilities. -
News Bavarian Nordic enters research collaboration to develop MRSA vaccine
The collaboration will use Exaxion's computer-based technology to discover novel antigens. -
News Teva and AbCellera enter into agreement to discover rare monoclonal antibodies
Agreement further supports Teva’s novel biologics development program. -
News New drugs from Abbvie, Gilead, Intercept and Merck forecast to achieve blockbuster status by 2020
Annual ‘Drugs to Watch’ study spotlights seven emerging blockbusters poised to enter the market in 2016. -
News Sanofi Pasteur to leverage its strong vaccine legacy in hunt for Zika vaccine
Building on the company's successful history in developing vaccines against similar viruses, most recently the introduction of Dengvaxia against dengue, Sanofi Pasteur is launching a Zika vaccine project. -
News BMS and Pfizer sign collaboration with Portola Pharmaceuticals to develop and commercialize investigational andexanet alfa in Japan
Andexanet alfa is designed to reverse the anticoagulant activity of Factor Xa inhibitors, including Eliquis (apixaban). -
News Aragen Bioscience announces ground breaking on state-of-the-art research facility
The new research space will add 15,000 sq.ft. to Aragen’s existing laboratories and will double capacity for diverse efficacy studies. -
News BMS and AbbVie receive positive CHMP opinion for investigational antibody, Empliciti, for the treatment of multiple myeloma
Positive opinion based on reduction in the risk of disease progression or death with Empliciti in combination with standard of care regimen for multiple myeloma demonstrated in ELOQUENT-2 study. -
News Allergan and AstraZeneca collaborate to develop and commercialize ATM-AVI for antibiotic-resistant gram-negative infections
ATM-AVI is an investigational, fixed-dose antibiotic combining aztreonam and avibactam. -
News Boehringer Ingelheim's Gilotrif demonstrated superiority to Iressa
Results of global Phase IIb LUX-Lung 7 trial demonstrate afatinib is superior in reducing the risk of lung cancer progression and the risk of treatment failure both by 27% compared to gefitinib. -
News Ixchelsis announces positive clinical proof of concept results for IX-01 in treating premature ejaculation
The drug was also shown to be extremely well tolerated with a safety and adverse event profile similar to that seen for placebo. -
News Fc receptor protein collection
Recombinant Fc Receptor proteins from AMSBIO are uniquely suitable for pharmaceutical research. -
News Shire resubmits NDA for lifitegrast to FDA
Resubmission includes positive data from OPUS-3, a Phase III efficacy and safety trial. -
News FDA accepts filing of cardiovascular outcomes data for Jardiance
Boehringer Ingelheim and Eli Lilly and Company expect to receive a decision from the FDA within the standard review time frame. -
News Valeant announces FDA acceptance of BLA submission for brodalumab in moderate-to-severe plaque psoriasis
The brodalumab BLA is supported by data from the three AMAGINE Phase III pivotal studies. -
News BMS's Opdivo + Yervoy regimen receives expanded FDA approval
Opdivo + Yervoy Regimen now indicated for unresectable or metastatic melanoma patients, regardless of BRAF mutational status, based on accelerated approval. -
News LDC enters new industry partnership for the discovery of novel medicines
LDC and Roche will jointly advance innovative drug discovery projects. -
News Shire completes acquisition of Dyax
The addition of Kalbitor and DX-2930 to Shire's portfolio strengthens its leadership position in hereditary angioedema. -
News Dynavax and AstraZeneca amend their agreement for asthma drug candidate
AstraZeneca to Conduct Phase IIa Clinical Study of AZD1419. -
News Lilly and Incyte submit NDA to FDA for oral once-daily baricitinib
Baricitinib is for the treatment of moderate-to-severe rheumatoid arthritis. -
News Sweeter treatment for breast and stomach cancers
Tweaking sugar molecules on anti-cancer antibodies improves cell-killing with fewer side effects. -
News Centauri Therapeutics to develop novel anti-infective therapeutics
Company has acquired Alphamer immuno-therapeutics platform technology. -
News AbbVie announces positive top-line results from second Phase III study investigating Elagolix in patients with endometriosis
Results show Elagolix reduces endometriosis-associated pain compared to placebo. -
News Boehringer Ingelheim and Arena Pharmaceuticals collaborate to advance research in schizophrenia
Collaboration partners to strengthen research in psychiatric diseases. -
News Sanofi Pasteur provides key support to the Human Vaccines Project
The research program is designed to accelerate the development of vaccines by decoding the human immune system. -
News New NIH awards will support development of therapeutic alternatives to traditional antibiotics
NIAID awards approximately $5 million in funding for 24 research projects. -
News FDA grants Priority Review for venetoclax NDA
Venetoclax, an investigational medicine, is a potential new way of treating the most common adult leukemia. -
News Scholar Rock announces option exercised by Janssen in immuno-oncology collaboration focused on supracellular activation
Company generates unique monoclonal antibodies that selectively inhibit the supracellular activation of latent TGF-beta 1 in the immune system. -
News HemoShear Therapeutics announces next stage of safety collaboration with Pfizer
Development of a biological and computational model for prediction of drug-induced vascular injury for early-stage compounds. -
News VR315 US completes clinical study
VR315 is a generic, inhaled combination therapy for asthma/COPD delivered using Vectura's proprietary dry powder inhaler and formulation technology. -
News Epigenomics receives FDA notification about status of pending approval decision for Epi proColon
Final approval of the company's application is subject to the resolution of minor outstanding topics, such as the use of appropriate language in the product labeling. -
News Regeneron and Sanofi announce sarilumab BLA Accepted for review by FDA
Sarilumab is an investigational, human monoclonal antibody intended for the treatment of patients with active, moderate-to-severe rheumatoid arthritis -
News Genomics and Vertex collaborate to identify target therapeutic pathways
Genomics’ proprietary statistical analysis tools and integrated multi-phenotype database to be used to support R&D at Vertex Pharmaceuticals. -
News Merck and Biocartis to collaborate on new liquid biopsy technology for RAS biomarker testing
Merck becomes first pharmaceutical company to collaborate with multiple diagnostic providers to support RAS biomarker testing. -
News Gilead terminates Phase II study of simtuzumab in patients with idiopathic pulmonary fibrosis
The study's Data Monitoring Committee recommended that the study be terminated early due to lack of efficacy. -
News MannKind and Sanofi to terminate Afrezza license and collaboration agreement
MannKind is now reviewing its strategic options for Afrezza -
News Merck, Pfizer and Syndax announce collaboration to evaluate combination of avelumab and entinostat in ovarian cancer
The three companies will collaborate to investigate safety, tolerability and preliminary efficacy of avelumab and entinostat in advanced ovarian cancer. -
News Epizyme announces first patient dosed in global clinical program evaluating tazemetostat in genetically defined solid tumours
US study sites now enrolling adults in registration-supporting Phase II study. -
News Cellectis files first clinical trial application for UCART19, for hematological malignancies
UCART19 is an allogeneic gene edited CAR T-cell product. -
News Teikoku Pharma USA, Inc. announces FDA approval of docetaxel injection
First non-alcohol formulation approved in the US. -
News Seattle Genetics and BMS announce initiation of Phase I/II clinical trial of Adcetris in combination with Opdivo in relapsed or refractory non-Hodgkin lymphoma
Second of two planned trials combining Adcetris and Opdivo under clinical collaboration agreement. -
News Actelion receices FDA approval of Uptravi for the treatment of pulmonary arterial hypertension
Uptravi will be made available to patients in the US in early January 2016. -
News Boston Therapeutics' sugardown in clinical trials reduces glucose, fructose and insulin
sugardown reduces total glycemic index including fructose by up to 28% and insulin by up to 18%. -
News Stellar Biotechnologies presents research on nanofiltration development at PepTalk Conference
The presentation reported data from the company's development and validation of filtration methods for the removal of viruses during manufacturing processes of KLH products. -
News APAC multiple myeloma therapy market hit $1.7 billion in 2014 and will add $1.1 billion by 2021
Emergence of novel therapeutics and minimal generic competition in China, Japan and Australia will drive APAC multiple myeloma market growth to 2021, says GBI Research. -
News Merck and Pfizer advance clinical development program with two additional phase III trials of avelumab
Merck-Pfizer Alliance achieves 2015 goal of initiating six pivotal trials with JAVELIN Ovarian 200 and JAVELIN Bladder 100 trials. -
News Boehringer Ingelheim's third generation EGFR TKI, BI 1482694, receives FDA Breakthrough Therapy Designation for treatment of patients with NSCLC
FDA designation reinforces potential for BI 1482694 to become a new treatment option for patients with EGFR mutation-positive lung cancer with a T790M mutation. -
News Bayer and CRISPR Therapeutics join forces to discover, develop and commercialize potential cures for serious genetic diseases
Bayer is investing USD 335 million in a long-term alliance with CRISPR Therapeutics through new Bayer LifeScience Center unit. -
News Increased investment spurs advances in gene therapy
The success of approved products for rare illnesses has opened the door for gene-based treatments of more prevalent diseases, finds Frost & Sullivan. -
News FDA Draft Guidance: Safety Assessment for IND Safety Reporting
The FDA has published draft guidance for sponsors of investigational new drug application (IND) studies with recommendations for identifying and evaluating important safety information that must be submitted to FDA and all participating investigators u... -
News Future obesity drugs must provide improved efficacy and safety, says GBI Research
A highly innovative and diverse pipeline of 248 products in active development is set to tackle the ever-encroaching obesity crisis. -
News WuXi and AstraZeneca form strategic alliance to expedite development of AstraZeneca's innovative biologics portfolio in China
Alliance will bring cutting-edge research and technical capability for biologics to China with the aim of addressing unmet patient needs in AstraZeneca's main therapy areas of respiratory, inflammation and autoimmunity; cardiovascular and metabolic dis... -
News Rexahn Pharmaceuticals announces new data for Supinoxin showing potent tumour growth inhibition
New data demonstrate Supinoxin dose dependently inhibits tumour cell growth in a preclinical model of triple negative breast cancer.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




























.jpg)












































.jpg)