- Home
- Biopharmaceutical Products
- Biopharmaceuticals (General Category)
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
Biopharmaceuticals (General Category)
Biopharmaceuticals (General Category) Companies (167)
Biopharmaceuticals (General Category) News
-
News Women in Pharma: Moving beyond discussions and into best practice at CPHI Milan
In this second CPHI Milan special of our monthly series, we cover the key takeaways from the Diversity & Wellbeing track held on October 10, 2024.31 Oct 2024 -
News Women in Pharma: A leader's approach to diversity advocacy
In our monthly series on women in the pharmaceutical industry, we interview leading experts in the pharmaceutical supply and value chain to discuss the importance of gender diversity in healthcare, the workplace, and beyond.20 Aug 2024 -
News Women in Pharma Anniversary: Celebrating Our Heroines of Pharma
Our Women in Pharma interview series is approaching its 1-year anniversary this month, and to celebrate, we are highlighting the Heroines of Pharma that our very own Women in Pharma admire.5 Jun 2024 -
News Drug Patent Expiries: a steep cliff or opportunity for innovation?
The pharmaceutical industry faces a patent cliff together in the years leading up to 2030. Learn what this means for drug pricing, their outsourcing partners, and drug innovation of the future.30 Apr 2024 -
News First offers for pharma from Medicare drug price negotiations
Ten high-cost drugs from various pharma manufacturers are in pricing negotiations in a first-ever for the US Medicare program. President Biden’s administration stated they have responded to the first round of offers.5 Apr 2024 -
News Special Edition Women in Pharma: Women’s health and the pharmaceutical industry
International Women’s Day is an annual celebration of women around the world every March 8. March also marks Women’s History Month. This March, our Women in Pharma series takes a look at how women’s healthcare has developed, and the r...25 Mar 2024 -
News Informa Markets International Women’s Day Panel Discussion
On March 7, 2024, the Informa Markets Amsterdam office hosted an International Women’s Day Breakfast and Panel Discussion to celebrate the women who drive the B2B events industry forward, including members of the CPHI team.8 Mar 2024 -
News Innovative Salts for Biopharma
With more than 135 years of experience, Dr. Paul Lohmann® is an expert in high quality Salt manufacturing and provides DPL-BioPharm Salts for upstream, downstream and fill & finish processes of the biopharmaceutical industry.24 Aug 2023 -
News Humira biosimilars at discounted prices to meet market demand for accessible treatment
Several biosimilars to AbbVie’s monoclonal antibody therapeutic Humira were launched by various pharmaceutical giants this week in the US, with more expected to be released by the end of the year as the patent expires.3 Jul 2023 -
News Biogen receives accelerated approval for first-of-its-kind ALS treatment
Biogen has announced that they have received approval from the US FDA for the amyotrophic lateral sclerosis (ALS) treatment, QALSODY™ (tofersen). This is the first approval of a treatment that targets a genetic cause of ALS.26 Apr 2023 -
News Eli Lilly gets ready to launch five new drugs in 2023
Eli Lilly, the American pharmaceutical company (IN, USA) are gearing up for a big year ahead, with hopes to launch five new drugs and capitalise on growing obesity and Alzheimer’s disease markets.15 Dec 2022 -
News From the floor - CPHI Frankfurt 2022
The live blog from the people on the ground at CPHI Frankfurt - covering all stand-out news, memorable moments, and keynote updates from the 2022 event.1 Nov 2022 -
News LG Chem to acquire AVEO Oncology with USD $566 million investment
South Korean chemical company LG Chem is set to acquire AVEO Oncology, an oncology-focused biopharmaceutical company based in the USA, as part of an investment to increase its footprint in North America and expand its development and commercialisation ...18 Oct 2022 -
News Thermo Fisher Scientific expands viral vector manufacturing capabilities with Plainville facility
Thermo Fisher Scientific’s new Plainville, MA viral vector manufacturing facility has opened, expanding the company's cell and gene therapy capabilities with advancing bioproduction and analytical instrumentation technologies.25 Aug 2022 -
News Lonza adds X-ray powder diffraction to micronisation portfolio in Monteggio (CH)
Lonza expands solid-state characterisation services for polymorph and solid-state properties of APIs with the addition of X-ray powder diffraction to its portfolio.25 Aug 2022 -
News Establishment of antiviral development centre for pandemic-level viruses
Researchers across several US institutions will come together to establish a centre for the research and development of antiviral drugs and therapeutics against pandemic-level viruses.10 Aug 2022 -
News Thermo Fisher Scientific announce expansion of cell culture media manufacturing site
The expansion of Thermo Fisher Scientific’s Grand Island, New York cell culture media manufacturing site offers increased redundant capacity in support of vaccine and biologic therapy development and manufacturing.5 Aug 2022 -
News mRNA production partnership to scale-up COVID-19 vaccine development
GreenLight Biosciences and Samsung Biologics announce mRNA production partnership to scale mRNA vaccine production to a commercial level with Samsung's expanded mRNA drug substance manufacturing site.3 Aug 2022 -
News The Japanese Pharma Market – Top Four Trends Shaping the Sector
Drug pricing, generics, investment, innovation - how these trends are shaping the face of Japanese pharma20 Jul 2022 -
News Investors turn attention to biotechs with clear path to market
Companies that are further down the road to market are more attractive to investors than those in early development29 Jun 2022
Biopharmaceuticals (General Category) Products (304)
-
Product ReadiLYCOAT® hydroxypropyl pea starch
ReadiLYCOAT® is a range of solutions providing immediate release coating to all kinds of tablets in various oral dosage forms in both pharmaceutical and nutraceutical applications. ReadiLYCOAT® products are mainly composed of hydroxypropyl pea starch. To ensure good film resistance over handling and storag...
-
Product DWRX1010 (Colonoscopy Bowel Preparation Drug)
※ DWRXs Differentiation Points 1) Maximize compliance in the form of minitablets. 2) Containing simethicone to remove foam in the colon, enabling smooth colonoscopy. 3) Maximizes the convenience of taking medication, and low water intake. ※Development Strategy 1) Non-clinical trial (KR) and patent applica...
-
Product CannEpil
CannEpil®, one of Argent BioPharma's flagship products, is an enhanced iteration of a compounded isolated cannabinoid formulation. It leverages well-known historical data to become one of the first compounded prescription investigational drugs used for seizure management over the past years. The active ing...
-
Product Biologic Quality Control and Release Testing
We deliver responsive QC analysis for complex biologic products from our cGMP laboratories. Our scientists develop and validate methods or perform technology transfer of a sponsor's method for a wide range of analytical methods required for batch release testing. We also routinely carry out testing to Phar...
-
Product Cortellis CMC Intelligence™
Cortellis CMC Intelligence™ product is a comprehensive database of guidelines / granular collection of CMC data requirements for initial registration of small-molecule and biologics drugs around the world that can be used to avoid delays in product approval and successfully bring a drug to the market (laun...
-
Product RoSS® Shell: Protection of single-use bags
Protect your single-use bioprocess containers: RoSS® Shells - abbreviated for Robust Storage and Shipping - reduce product loss towards 0%. Compatible with all available sizes and types of single-use bags, RoSS enables standardized and scalable end-to-end process solutions for fluid management and col...
-
Product HANQUYOU(trastuzumab)
HANQUYOU (trastuzumab, trade name: HERCESSI™ in the U.S., Zercepac® in Europe) was successfully launched in the U.S., China and Europe, becoming the Chinese mAb biosimilar entering the U.S., the EU and China market. It is indicated for the treatment of HER2 positive early breast cancer, metastatic bre...
-
Product Safe and cost-efficient packaging of pharmaceutical products
After introducing a new medication to the market, it is always a challenge for pharmaceutical manufacturers to calculate production capacities without incurring economic losses. With support from machine manufacturer Schubert-Pharma and packaging specialist Faller Packaging, manufacturers now have two stro...
-
Product Aprotinin
DADELI is a GMP-certified pharmaceutical manufacturer for intermediates,APIs and finished formulations.Our main APIs include HCG,Aprotinin(Currently in China we are the only one to produce Aprotinin complying with the latest EP 11.0), Oxymatrine,Isoniazidum,Egg Lecithin,Daidzein,etc.Warmly welcome all cust...
-
Product BioPharma
When you know the biopharma solution you need and want to find the perfect delivery partner, Caldic has the expertise to source, process and customize packaging to your exact requirements. From packaging and single use solutions to stainless steel vials, and following allergen, GMO or REACH regulatory requ...
-
Product Biosimilars Portfolio
• Pegfilgrastim (Filed with EMA) • Filgrastim (Filed with EMA) • Trastuzumab (Filed with EMA, MHRA, Received recommendation for marketing authorization in India) • Bevacizumab (Filed with MHRA) • Omalizumab (Global PhIII ongoing) • Denosumab (Global PhIII ongoing) • Ranibizumab (Global PhIII ongoing...
-
Product Excipient Grade Sodium Hyaluronate (Hyaluronic Acid)
Excipient Grade Sodium Hyaluronate has the registration of pharmaceutical excipients and with high stability. It has the advantages of ultra-low impurity level, ultra-low endotoxin level, ultra-low metal impurity residue and ultra-low microbial pollution.
• Microbia...
-
Product BioHale® Trehalose Dihydrate
BioHale® Trehalose Dihydrate is a non-reducing disaccharide consisting of two glucose molecules which are linked by an α, α-1,1 bond. It is used in the pharmaceutical industry as a stabilizer of biologics.
Trehalose Dihydrate has proven to be highly effective in protecting cell membranes...
-
Product N3-PEG11-NH2
Azido-PEG11-amine (CAS No. 1800414-71-4) is a monodispersed PEG derivative containing an amino group with an azide group. The azide group can react with alkyne, BCN, DBCO via Click Chemistry to yield a stable triazole linkage. The amino group is reactive with carboxylic acids, activat...
-
Product Multicompendial TRIS AMINO and TRIS Amino Ultra Pure reagents
Advancion is the world’s only fully integrated manufacturer of globally compliant TRIS AMINO™ reagents for use in a variety of demanding pharmaceutical and biotechnology applications. With more than 50 years of TRIS manufacturing experience, we continue providing TRIS AMINO grades that meet the dynamic nee...
-
Product Tris(Hydroxymethyl)Nitromethane CAS 126-11-4
CAS NO::126-11-4Appearance::White To Almost White Powder
Molecular Formula::C4H9NO5
Molecular Weight::151.12
EINECS NO::204-769-5
MDL NO::MFCD00007395
-
Product Supply Chain Management
• GMP Compliant Storage of a wide range of client products from API and excipients to consumables as well as both Stability and Retention sample programs. • Facility and Staff certified for storage, handling, and shipment of hazardous materials. Including Flammable, Toxic, Potent, etc. products. • Envir...
-
Product Tannic acid
Name: Tannic Acid
Alias: Tannins
CAS #1401-55-4Molecular Formula: C76H52O
Appearance:Light brown irregular powder

-
Product Hoses and Hygenic Connections
Our hoses for pharma biotech, cosmetics, food, beverage and chemical industry
We offer you a wide range of hoses specially designed for the pharmaceutical, biotech, cosmetic, food, beverage and chemical industries. We also ensure their proper use through training in the co...
-
Product Tris Hydrochloride low endotoxin, pharma grade CODE 633654
https://www.itwreagents.com/iberia/en/product/tris-hydrochloride-low-endotoxin-pharma-grade/633654
-
Product Ready-to Use CHO cell lines
UGA Biopharma GmbH offers to their clients so called Ready-to-use Biosimilar Cell Lines. This are in-house developed CHO cell lines stably expressing different biosimilar molecules, that are instantly available for in-licensing. The client profits, because the cell lines are directly available, and develop...
-
Product Bioprocessing solutions and excipients for protein therapeutic formulations
From excipients for protein therapeutic formulations, to innovating solutions for bioprocessing, we have decades of experience in protein delivery. TALK TO US about our latest launches: Virodex™ detergent range, a sustainable alternative to Triton™ X-100 for viral inactivation and cell lysis. Plus, Tween...
-
Product MAST® Autosampling Solution
A Versatile & Modality-Agnostic Aseptic Autosampling Solution for Reliable On-Line and Near Real-Time Sample Analysis
-
Product Sodium Hyaluronate - Injection grade
Hyature® is a pharmaceutical grade sodium hyaluronate which can be used as an API or excipient for drugs and medical devices in ophthalmic preparations, intra-articular injections, anti-adhesive preparations, topical preparations for wound healing and soft tissue filler, etc. Hyature® sodium hyalur...
-
Product Fluid Transfer
A full range of tubing and hoses best suited for the biopharma and pharmaceutical markets. From TPE to silicone, each material type offers a unique set of features and benefits. ValPlus™, an enhanced level of tubing validation certification, is available for select formulations.
-
Product GMP Liquid Buffer & Custom Liquid Blends
Actylis manufactures buffers, process solutions, cleaning solutions, Water for Injection (WFI) for further processing, and custom liquid blends in complinace with Good Manufacturing Practices in our new state-of-the-art GMP liquid processing facility in Ireland.
Actylis has seven manufacturing facil...
-
Product Anti-tuberculosis vaccine BCG10
BCG 10 Anti-Tuberculosis Vaccine is used for protective vaccinations against tuberculosis. Catch-up (supplementary) vaccinations in subjects unvaccinated after birth should be performed as early as possible, no later than at 15 years of age. In case of BCG immunisation booster vaccine doses are not recomme...
-
Product Sterility Test Isolator
1. Built-in Vaporized Hydrogen Peroxide (VHPS) generator for bio-decontamination which can reach 6-log Sterility Assurance Level
2. The VHPS residual concentration is less than 1ppm after aeration
3. leakage rate is less than 0.5% /vol/hour under 2 times working pressure test
qq...
-
Product Tofflon Integrated Solution for Biologicals MFG
Applications: Biosimilars& Therapeutical Protein Nucleic Acid-based Vaccine(mRNA/DNA) Recombinant Viral Vector Vaccine Inactivated/Attenuated Viral Vaccine Gene Therapy

-
Product Gamma Irradiated Cleanroom Facemasks With Tyvek Side Extensions
Gamma Irradiated, Extra Large Cleanroom Facemask with Tyvek® Side Extensions, Green or Blue, with Double Elastic Headbands and with or without Fog-Prevention Adhesive are made from an inner facing of soft cellulose material, an outer facing of spunbonded polypropylene and a 100% meltblown polypropylene med...
-
Product CDMO
• Cytotoxic Injection (Liquid , Lyophilized) • ONCOLOGY • CYTOTOXIC API • EU GMP • JGMP (Japan) • KGMP (Korea) • PIC/S
-
Product Biopharmaceutical Analysis
Solvias has a solid reputation with market-leading biopharmaceutical companies for the characterization and QC analysis of monoclonal antibodies (mAbs), glycoproteins, PEGylated proteins and peptides and biosimilars (follow-on biologics).. This means that businesses of all sizes, even smaller biotechs, can...
-
Product WHEATON Roller Apparatus by DWK
WHEATON® roller apparatus are used for roller bottle cell culture, an established cell culture method and relatively inexpensive way to set up a flexible and scaleable biopharmaceutical operation. In addition to the standard product DWK also offer a wide range of customized options to accommodate the most ...
-
Product Proton drops
Rickets prophylaxis.
DOSAGE8 drops (400 I.U.) per day for a 18-months period.1 drop contains: 50 I.U. of Vitamin D3.
INGREDIENTS
Vitamin D3 (Cholecalciferol).
CHARACTERISTICSMicellar solution in physiological solution easy to be mixed with all liquids or foods. Tasteless...
-
Product Sterile Grade at ISO Class 5 facility
Kikkoman can offer "sterile grade” products, purified through a super purification process at our ISO Class 5 facility, approved by the Japanese Authority.
-
Product Gx RTF® ClearJect® Polymer syringes
Gx RTF® ClearJect® syringes are made from COP (Cyclo Olefin Polymer). They are glue-free as the cannula is not glued in, but is instead insert-molded during the injection molding process, no aggregation due to metal or tungsten residues, high viscosity silicone oil for reduced particles, minimal extractabl...
-
Product Icatibant
is a synthetic peptidomimetic drug consisting of ten amino acids, and acts as an effective and specific antagonist of bradykinin B2 receptors. It has been approved in the EU for use in hereditary angioedema, and is under investigation for a number of other conditions in which bradykinin is thought to play ...
-
Product Biotech Development
Nordmark Arzneimittel GmbH & Co. KG provides a wide range of biotech services such as strain (including MCB and WCB Production) and process development - including special experience in microbiom formulation - up to clinical phase III. Contact us for more information.
-
Product Antide
CAS No. : 112568-12-4.Molecular Formula: C82H108ClN17O14 Application: Anti-microbial Main product: Oxytocin Acetate,Vasopressin Acetate,Desmopressin Acetate,Terlipressin acetate, Caspofungin acetate, Micafungin sodium, Eptifibatide acetate, Bivalirudin TFA, Deslorelin...
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product 4-Fluoro-7-(Methylsulfonyl)-2,3-Dihydro-1H-Inden-1-One II Belzutifan Internediate II CAS: 1672665-29-0
4-Fluoro-7-(Methylsulfonyl)-2,3-Dihydro-1H-Inden-1-One II Belzutifan Internediate II CAS: 1672665-29-0
-
Product Tyramine
Synonyms: 4-(2-aminoethyl)phenol CAS: 51-67-2 Molecular Formula: C8H11NO Molecular Weight: 137.18 Properties: White to off-white powder
-
Product Liveo™ Pharmaceutical-Grade Tubing
These high-purity tubings are available in both platinum-cured silicone and thermoplastic elastomer (TPE) options and are designed for ultra-pure fluid transfer applications in pharmaceutical and biotechnological manufacturing processes.
With more than 30 options to choose from, they are t...
-
Product PBP1510 (Ulenistamab) Anti-PAUF Antibody for Pancreatic Cancer
Ulenistamab (PBP1510) is a First-in-Class antibody drug developed as a target of pancreatic adenocarcinomaup-regulated factor (PAUF) resulting in decreased autocrine and paracrine signaling implicated pancreatic cancer.
It attenuates the tumorigenic potential of PAUF in both the pancreatic cancer ce...
-
Product Gene Therapy Services
Our gene therapy process development and manufacturing capabilities are designed to confidently and rapidly progress your products from gene to market through development of robust and reproducible cGMP practices.
-
Product Recombinant Trypsin
In compliance with pharmacopoeia standards: in compliance with relevant standards for recombinant proteases in pharmacopoeias.No animal origin: produced by recombination, free of exogenous viral contamination, and no animal-derived materials are used in the production process.
Quality stability: mass pr...
-
Product Diphenylpyralin HCL
The established active pharmaceutical ingredient (API) has been long proven as an antihistamine, analgesic and nasal decongestant. Kraeber & Co supplies the synthetic active ingredient exclusively: • FDA and GMP certifi ed German production • New process development • High purity • Soluble in water or ...
-
Product Packing biotech products
Rommelag CMO has a separate facility for filling all biological pharmaceuticals up to biosafety level 2 (BSL-2) – including active pharmaceutical ingredients (API) such as vaccines and antibodies. This is one of the few blow-fill-seal production facilities in the world to meet this standa...
-
Product Human Histaglobulin
The main component of this preparation is human histaglobulin, of which the human immunoglobulin is originated from human plasma from healthy donors. The manufacturing process contains a step of low pH incubation for viral inactivation. It contains suitable amounts of sodium thiosulfate and glucose a...
-
Product Sterile Manufacturing
Avara Pharmaceutical Services offers liquid and lyophilized fill-finish of sterile injectable products from state-of-the-art facilities in North America and Europe. Contact us for more information.
-
Product Bioinformatics
We offer integrated bioinformatics services to biotech and pharmaceutical companies across the drug discovery pipeline. Our bioinformatics services span the entire gamut from building data packages, pathway databases and knowledge bases, to analysis of high throughput data, predictive modeling, structural ...
-
Product Guanidine Thiocyanate Excipient and Intermediate
BioSpectra offers a wide range of products, which includes guanidine thiocyanate. Features: it is a chaotropic agent. Contact us for more information.
-
Product Low molecular weight chondroitin sulfate - tea
Syntex manufactures a wide range of active pharmaceutical ingredients, including sodium heparin both from bovine and porcine origin, calcium heparin, lithium heparin, low molecular weight heparin and pyrogen free chondroitin for injectables. Syntex complies with GMP and exports to Annex I countries, such u...
-
Product Hemp & Cannabis Processing
InCon has special technology for the Cannabis & Hemp purification market. With over 25 years of high vacuum distillation technology experience, InCon Process Systems has designed, built, and operated the world's largest Wiped Film & Short Path Distillation plants for hundreds of different compounds...
-
Product Biological Activity
This test provides a direct measure of the effectiveness of the active ingredient.
-
Product Biosimilars Testing Services
Testing services for biosimilars: Our biosimilar analytical comparability programs provide highly relevant data for early stage characterisation and later stage comparison. These programs evaluate and compare all pertinent features of the biosimilar product and are based on the ICH Q6B. Programs encompa...
-
Product Antibody / Monoclonal antibody therapeutics services
Monoclonal antibody therapeutics: We continually invest in advanced analytical instrumentation that allows us to deliver mAb analytical data with the highest degree of sensitivity, accuracy and resolution. Our experience spans recombinant monoclonal antibodies and related products such as biosimilars, ...
-
Product Protein Aggregation Analysis and Characterization
Analysis and characterization of protein aggregates: The services provided by our experts include a range of protein aggregation analysis techniques that are used to detect and quantify aggregates in solution in support of formulation development, quality control, stability studies, comparability, rel...
-
Product cGMP Cell-based Bioassays
Cell-based bioassays that comply with cGMP: Using a variety of techniques, including cell-based assays, ligand and receptor binding assays, and flexible multiple assay approaches – our potency testing experts offer tailored bioassay method development, method transfer and method validation to ICH Q2 (R1)...
-
Product Oligonucleotide Analytical Development Services
Analytical development services for oligonucleotides: For oligo-based drugs, our capabilities range from GLP bioanalysis to GLP/cGMP characterisation. We support product development, from quality control testing of amidite starting materials and early stage product characterization through to GMP batch r...
-
Product HANSIZHUANG (Serplulimab Injection)
HANSIZHUANG (recombinant humanized anti-PD-1 monoclonal antibody injection, generic name: serplulimab injection) is the first anti-PD-1 mAb for the first-line treatment of SCLC and has been approved in China and Indonesia. Up to date, 4 indications are approved for marketing, 2 marketing applications a...
-
Product Chorionic Gonadotrophin (HCG)
DADELI is a GMP-certified pharmaceutical manufacturer for intermediates,APIs and finished formulations.Our main APIs include HCG, Aprotinin(Currently in China we are the only one to produce Aprotinin complying with the latest EP 11.0), Oxymatrine,Isoniazidum,Egg Lecithin,Daidzein,etc.Warmly welcome all cus...
-
Product Injection Grade Sodium Hyaluronate (Hyaluronic Acid)
Injection Grade Sodium Hyaluronate has GMP certification in China and the status of API registration status is A. It has the advantages of ultra-low impurity level, ultra-low endotoxin level, ultra-low metal impurity residue and ultra-low microbial pollution.
· Microbial ...
-
Product Eye drop Grade Sodium Hyaluronate (Hyaluronic Acid )
Eye-drop Grade Sodium Hyaluronate has GMP certification in China and the status of API registration status is A. It has the advantages of ultra-low impurity level, ultra-low endotoxin level, ultra-low metal impurity residue and ultra-low microbial pollution.
• M...
-
Product Medical Device Grade Chondroitin Sulfate Sodium
Medical Device Grade Chondroitin Sulfate Sodium extracted from shark bone as raw material, fish source is more similar to human DNA and easily absorbed by the human body. And Enzymolysis by special enzymes, is harmless to the human body, and will enzymatic hydrolysis of excess proteins. It can be pur...
-
Product Biochemicals, Biomolecules and Salts
At Advancion, we consistently deliver products through a traceable and auditable supply chain of high-quality fine chemicals tailored to help you successfully move your products from pilot to commercial-scale production. Choose with confidence from our selection of product offerings incl...
-
Product Nitroalkanes and derivatives solvents and building blocks for pharmaceutical synthesis
The effectiveness of nitroalkanes lies in their ability to provide alternative synthetic routes to existingcompounds, as well as highly efficient routes to new compounds. The exceptional versatility and highreactivity of nitroalkanes provide a means to conduct synthetic transformations under mild condition...
-
Product HEPES and HEPES Sodium Salt Buffers
Our latest investment in new manufacturing capacity significantly expands our portfolio of in-house-produced biological buffers and moves Advancion into a leading global supply position for the HEPES molecule. HEPES Biologics Plus grade buffer products are produced by Advancion in its Sterlington facility ...
-
Product 2,3,4-Trihydroxybenzaldehyde
Name: 2,3,4-Trihydroxybenzaldehyde
CAS No: 2144-08-03
EINECS No.:218-404-2
Chemical Name: Pyrogallolaldehyde
Structure Formula:
Molecular formula&Molecular Weight: C7H6O4; 154.1201
...
-
Product Methyl 3,4,5-trimethoxybenzoate
Name: Methyl 3,4,5-trimethoxybenzoate
CAS No: 1916-07-0
Chemical Name: 3,4,5-Trimethoxybenzoic acid methyl ester
Structure Formula:
Molecular formula&Molecular Weight: C11H14O5; 226.2259
Appearance...
-
Product Pyrogallol
Product Name:Pyrogallol
Alias: Pyrogallic acid, 1, 2.3-trihydroxybenzene
CAS No: 87-66-1
Molecular Formula:C6H6O3;
Molecular Weight:126.11
Appearance: White glossy crystalline powder.
Storage: Moisture-proof and a...
-
Product N-Acetyl-DL-Tryptophan (Ph. Eur, BP) low endotoxin, pharma grade CODE 637763
https://www.itwreagents.com/iberia/en/product/n-acetyl-dl-tryptophan-ph-eur-bp-low-endotoxin-pharma-grade/637763
-
Product Mobius® Gold High Cell Density Cryopreservation Assemblies (-80 °C)
A single-use assembly designed to facilitate the freeze and thaw of cryopreserved cells at -80 °C. Mobius® Gold High Cell Density Cryopreservation Assemblies enable seed train inoculation with cells at a higher cell density and larger volume, which significantly accelerates the cell expansion process. The ...
-
Product Cellicon® Perfusion Filter and Controller
A Superior Cell Retention Solution for N-1 Seed Train Intensification with Optimized Process Control. The benchtop Cellicon™ Perfusion Solution is designed to meet your perfused seed train challenges. It consists of a controller and a flat sheet cell retention filter with a single-use assembly running in t...
-
Product Cellicon® Cell Retention Solution for Process Scale
Increase Your Bioreactor Productivity with our superior solution for upstream process intensification. In combination with your bioreactor, the Cellicon® Cell Retention Solution allows you to achieve significantly higher viable cell densities, improving your upstream productivity and manufacturing flexibil...
-
Product Ultimus® Film
An innovative single-use film offering strength and leak resistance for your toughest liquid bioprocessing applications. Ultimus® film provides enhanced bag strength, improved durability and leak resistance through a novel strength layer reinforced by woven nylon. The inert fluid contact layer promotes hea...
-
Product Novaseptum® SURe Sterile Sampling Assemblies
A single-use sterile sampling assembly that takes representative samples while protecting your bioprocess from cross-contamination. NovaSeptum® SURe sampling assemblies can be easily integrated into your single-use manufacturing process. Available in five popular container formats and with two connect...
-
Product Mobius iFlex® Bioreactor
The next generation of high performance single-use modular bioreactors for fed-batch and perfusion processes
-
Product ProCellics™ Raman Analyzer with Bio4C® PAT Raman Software
An Easy-to-Use GMP Process Analytical Technology (PAT) Platform to Monitor Cell Cultures In-Line and in Real-Time, from Process Development to Manufacturing
-
Product Pellicon® Capsules with Ultracel® Membrane
Accelerate your therapy using our innovative single-use tangential flow filters with superior flux performance. Ideal for single-use tangential flow filtration (TFF), Pellicon® Capsules provide high flux and linear scalability in a plug ‘n play format for optimum process flexibility and productivity. Pelli...
-
Product MilliStak+® Depth Filters
Innovative, High-Performance Pod Filters are Ideal for Primary and Secondary Clarification in Lab, Pilot and Process-scale Applications. Millistak+® depth filter media is offered in a scalable, disposable format: the Pod Filter System.
-
Product Natrix® Q Chromatography Membrane
Natrix® Q Chromatography Membrane for single-use, scalable purification, Natrix® chromatography membrane is a high capacity, high throughput strong anion exchange device designed to purify biomolecules. It combines the capacity of high performing resins with the exceptional flow properties of chromatograph...
-
Product Millistak+® HC Pro Synthetic Depth Filter
Millistak+® HC Pro (high-capacity synthetic media) is a family of synthetic depth filters providing a cleaner and more consistent depth filtration media over current diatomaceous earth (DE) and cellulose (CE) based filter offerings. Multiple media grades are available for primary and secondary clarificatio...
-
Product Hyatrue® — Pharmaceutical Grade Sodium Hyaluronate
Hyatrue® is a pharmaceutical grade sodium hyaluronate which can be used as an API or excipient for drugs and medical devices in ophthalmic preparations, intra-articular injections, anti-adhesive preparations, topical preparations for wound healing and soft tissue filler, etc. Hyatrue® sodium hyaluronate ...
-
Product Onko BCG 100
BCG for immunotherapy.
The product is intended for treatment of superficial, epithelial non-invasive urothelial carcinomas (carcinoma urotheliale Ta, Tis, T1)
1 ampoule or 1 vial with the powder contains:
Live attenuated bacilli Bacillus Calmette-Guerin, Brazilian BCG Moreau substrain ...
-
Product UV Light Blocking Tyvek Equipment Covers
UV Light Blocking Tyvek Equipment Covers are designed to keep cleaned pharmaceutical equipment and supplies clean and protected from external sources of contamination while also providing in-process protection to light sensitive biotech products.
-
Product Sodium Hyaluronate - Medical grade
Hyature® is a pharmaceutical grade sodium hyaluronate which can be used as an API or excipient for drugs and medical devices in ophthalmic preparations, intra-articular injections, anti-adhesive preparations, topical preparations for wound healing and soft tissue filler, etc. Hyature® sodium hyaluronate is...
-
Product Innovation Hub_Pharma Virtual Lab by Roquette
Discover how we can help you Roquette as a technology partner and a trusted supplier, provides scientists and researchers with a collaboration platform to beter understand and formulate with our ingredients. Innovation Hub | Roquette Pharma Solutions
-
Product FPFs
• Genexol®PM Inj.(Paclitaxel) • Genexol® Inj.(Paclitaxel) • Nanoxel®M Inj.(Docetaxel) • Docetaxel Inj.(Docetaxel) • Nexatin® Inj.(Oxaliplatin) • Everose® Tab.(Everolimus) • Pemed®S Inj.(Pemetrexed) • Azalid® Inj.(Azacitidine) • Decilid® Inj.(Decitabine) • Lenalid® Inj.(Lenalid...
-
Product Protein Analysis and Charactersation
Our protein analysis scientists provide characterization services in accordance with the ICH Q6B Guidance to ensure the quality and consistency of your product. With strengths in protein structure analysis including higher order structure, physiochemical property determination, bio...
-
Product Protein or Biologics Higher Order Structure Analysis
We provide higher-order structure analysis to drive insight into the secondary and tertiary structures of protein in “normal “presentation as well as a function of temperature and pH, allowing the study of subtle structure changes that may occur during processing, storage or handling as well as protein agg...
-
Product Comparability Studies for Biopharmaceuticals
We design bespoke analytical programmes that ensure that the relevant quality attributes for your drug substance or product are evaluated to support your manufacturing process changes. We select analytics referenced by the ICH Q6B to examine the product's structural features (primary, secondary and&nb...
-
Product Isolation and Characterization of Product-related Impurities
Intertek offers product-related impurity analysis in line with ICH Q6B with laboratory-scale isolation and a range of chromatography or mass spectrometry approaches. Our experienced scientists perform detailed characterization using a diverse range of technologies which include MALDI-MS, LC-MSMS, HPLC...
-
Product Ligand-binding Assays
Intertek offers wide range of pharmaceutical services which includes ligand-binding assays. It belongs to immunochemistry services category. It includes elisa, electrochemiluminescence (ecl), radioimmunoassay (ria), etc.
To learn more, visit our website:
www.intertek.com/pharmaceut...
-
Product Variable Volume Micropipettes by CAPP
Ecopipette line of single-channel pipettes is designed to take manual liquid-handling workflows to a whole new level.Ecopipette autoclavable pipettes are crafted from the best materials, each single-channel pipette features a fully autoclavable body (except for the volume controller knob) to minimize the t...
-
Product KLEPTOSE® HP HPB - Hydroxypropyl betacyclodextrin
Kleptose HP and HPB for pharmaceutical applications: parenteral use, syrup oral suspensions. HPBCD increase water solubility and bioavailability.
-
Product Sisonal Solution
Useful to promote the physiological functionalities of the respiratory system and to stimulate the immune defences.
DOSAGEChildren: 5 ml twice a day.Adults: 5 ml three times a day.
INGREDIENTSEchinacea angustifolia DC roots E.S. tit. Min. 4% in echinacoside, Propolis minced ...
-
Product Redox Drops
Redox drops is an antioxidant, it performs a protective action against free radicals. Vitamin C contributes to the normal functioning of the immune system, to cells protection from oxidative stress and it improves iron absorption.
DOSAGETake 10 – 20 drops (100 – 200 mg of Vitamin C) per day unle...
-
Product Proton Tablets
The cholecalciferol contributes to normal absorption and use of calcium and phosphorus, the normal function of the immune system and the maintenance of normal muscle function.
DOSAGE
1 tablet a day.
INGREDIENTS
Cholecalciferol (Vitamin D3).
CHARACTERISTICS
Vitamin ...
-
Product Proton DK 50 Drops
Rickets prophylaxis and Vitamin K deficiency bleeding (VKDB).
DOSAGE6 drops per day until the bottle is empty.6 drops contains 400 I.U. of Vitamin D3 + 50 mcg of Vitamin K1.
INGREDIENTSVitamin D3 (Cholecalciferol) + Vitamin K1 (Phylloquinone).
CHARACTERISTICSMicellar soluti...
-
Product Patux solution
Patux solution thanks to its composition studied for a rapid and effective action, is useful in case of dry cough.
DOSAGEChildren above 2 years: 2,5 ml three times per day. Adults: 5 ml three times per day.
INGREDIENTSAlthea officinalis roots E.S., Plantago major L., Marrubi...
-
Product Panavit solution
Prophylaxis and therapy of the vitamin deficiencies. Dietary supplement in the infant’s alimentation.
DOSAGEPreterm infant : 10 drops per day for one year.In term infant : 5 drops per day for a 18-months period.
...
-
Product Panavit Drops + Zinc
Panavit drops is a balanced multivitamin dietary supplement, useful to fill nutritional deficiencies or increased needs of the nutrients contained therein. Vitamin D3 contributes to the normal absorption and use of calcium, phosphorus and to the normal function of the immune system. Zinc contribu...
-
Product Normuril solution
Normuril solution is a dietary supplement of Cranberry and Propolis useful for the physiological function of the urinary tract with a pleasant taste of berries.
DOSAGE
Daily dose: 2 0 ml diluted in a glass of water.
Impact dosage: 20 ml diluted in a glass of water prefe...
-
Product Naf Spray
Fluorine prophylaxis of the dental caries.
DOSAGEfrom 2 to 4 years: 0,50 mg per day (2 puffs).from 4 years and in adults: 1mg per day (4 puffs).1 puff contains 0,25mg of Fluorine ions (F-).
INGREDIENTSFluorine + Xylitol (Sodium Fluoride and Xylitol).
CHARACTERISTI...
-
Product Naf Drops
Fluorine prophylaxis of the dental caries.
DOSAGEfrom 0 to 2 years : 8 drops (0,25 mg) per day.from 2 to 4 years : 16 drops (0,50mg) per day.8 drops contain 0,25 mg of Fluorine ions (F-).
INGREDIENTSFluorine + Xylitol.
CHARACTERISTICSFluorine and Xylitol in watery...
-
Product Lipovit drops
Vitamin A, Vitamin D3, Vitamin E and Vitamin K1 dietary supplement. Useful to fill the nutritional deficiencies or the increased needs of these nutrients.
DOSAGE5 drops per day unless otherwise medical advice.5 drops contain: Vitamin A = 1 mg (= 1000 RE = 3333 I.U.)Vitamin D3 = 10 mcg (400 I.U.)...
-
Product Lioflor drops
Lioflor® drops is a dietary supplement based lactic species Bifidobacterium animalis ssp lactis BLC1, probiotic belonging to the family of Bifidus, present in the intestinal flora of man. The Bifidobacterium animalis ssp lactis BLC1 is able to colonize the intestine and promote the balance of intestin...
-
Product Lioflor caps
Lioflor caps is a dietary supplement of probiotics, useful to help the balance of intestinal flora, also indicated as an adjunct in the prevention of relapsing forms of vaginosis, vaginitis and infections of the lower urinary tract.
DOSAGE
1 caps a day between meals, unless medically indica...
-
Product K start drops
Vitamin K deficiency bleeding prophylaxis. (VKDB)
DOSAGE10 drops (50mcg) per day for 2 bottles.
INGREDIENTSVitamin K1 (Phylloquinone).
CHARACTERISTICSMicellar solution in aqueous phase, easy to be mixed with all liquids or foods. Tasteless and odorless.

-
Product Jo Oil oily bath
Oily bath is the ideal detergent for sensitive, dehydrated or atopic skin. Its composition is rich in substances of vegetable origin such as Jojoba oil, and can be used daily on delicate skins for maintaining a correct skin hydration.
DOSAGE
6 sprays (about 10 ml) are sufficient for the was...
-
Product Jo 18 Cream
For its composition JO18 results ideal for soothing inflamed skins, atopic and seborrheic dermatitis, sunburn and irritation in general.JO18 allowing an activity:
• Lenitive;
• Minimizing the itch;
• Strengthener the skin functionality;
• Stimulating the skin’s p...
-
Product Idrofolic plus drops
Useful in the treatment of pernicious anemia and certain diseases of the nervous system. It is able to lower the plasma levels of homocysteine.
DOSAGE
8 drops per day unless a prescription.
1 drop contains 50 mcg of folic acid and 4 mcg of cyanocobalamin.
INGREDIENTS q...
-
Product Idrofolic drops
Hematological disorders. Prevention of the defects of the neural tube.
DOSAGE5-10 drops per day unless otherwise medical advice.1 drop contains: 40 mcg of Folic Acid.
INGREDIENTSFolic Acid (Vitamin B9).
CHARACTERISTICSIdrofolic drops is a dietary supplement of Folic Acid in...
-
Product Folium solution
Anemia caused by a lack of folates.
DOSAGE1-4 ml per day.
INGREDIENTSFolic Acid (Vitamin B9).
CHARACTERISTICS1 ml corresponds to 100 mcg of Folic Acid.Strawberry taste.

-
Product Folium 400 tablets
Anemia caused by a lack of folates.Prevention of the defects of the neural tube.
DOSAGE½ – 1 tablet per day.
INGREDIENTSFolic Acid (Vitamin B9).
CHARACTERISTICS1 tablet is equal to 400 mcg of Folic Acid.

-
Product Ferrosil Plus solution
Ferrosil Plus solution is a dietary supplement of iron highly absorbable and pleasant taste because microencapsulated in liposomes. Iron absorption is increased by the presence of Vitamin C.
DOSAGE
Take 5 – 10 ml of Ferrosil plus solution equal to 20/40 mg of elemental iron daily as needed ...
-
Product Ferrosil Mom
It is indicated to contrast a form of anemia in pregnancy that takes the name of gestational iron-deficiency anemia.
DOSAGE
Take 5 – 10 ml of Ferrosil Mom solution equal to 20/40 mg of elemental iron daily as needed preferably with meals, unless otherwise specied by the doctor. 5 ml contain 20 mg...
-
Product Ferrosil drops
Iron-deficiency anemia.
DOSAGE2 drops (1 mg) /Kg/dieor 1 drop (0,5 mg) / ½ Kg/die.2 drops contain: 1 mg of elementary Iron.
INGREDIENTSGluconate Iron (Fe++).
CHARACTERISTICSGluconate iron in watery solution, easy to be mixed with all liquids or foods.

-
Product Eucolin drops
Thanks to its composition appositely studied for a rapid and effective action, Eucolin drops results useful in states of the infant’s gaseous colic and in the abdominal pains of spastic nature.
DOSAGEInfant’s gaseous colic: 10 drops from one to three times per day, half an hour before the b...
-
Product Ergon vials
Ergon vials is a dietary supplement of Iron, Folic Acid, Vitamin B12 and Vitamin C, useful to fill nutritional deficiencies or increased needs of these nutrients. Iron contributes to normal transport of oxygen in the body, folic acid and Vitamin B12 contributes to the normal formation of red blood cells ...
-
Product Equidral sachets
Equidral sachets is a dietary supplement indicated in the treatment of the dehydration caused by acute diarrheas, altered electrolytic equilibrium, vomit, gastroenteritis, hyper-sweating.
DOSAGEDissolve the content of a sachet each 250ml of water and shake until the solution is well mixed. Admin...
-
Product Ecevit drops
Ecevit drops is a dietary supplement of alfa-tocopheril acetate (Vitamin E), used to fill a nutritional deficiency or to increase the requirements of this nutrient. Vitamin E helps protect cells from oxidative stress.
DOSAGETake from 1 to 6 drops per day according to the needs. Do not exceed rec...
-
Product Normociclo tablets
Normociclo is a dietary supplement based on plant extracts (pilosella, turmeric, agnocasto and ortosifon), with Magnesium and bromelain. Pilosella and ortosifon are useful for draining body fluids; turmeric and chaste counteract disorders of the menstrual cycle, while Magnesium contributes to electrolyte b...
-
Product Destrolac solution
Treatment of the constipation in children or adults.
DOSAGEUnless otherwise medical advice:Children: 10 ml per day, preferably in the evening.Adults: 20 ml per day, preferably in the evening.
INGREDIENTSMaltodextrine 72%, Bioecolians® (prebiotic) 10%, Sugar (saccharose) 10%,...
-
Product Destrolac powder
Dietetic treatment of the constipation.
DOSAGE3 measuring cups (5g) every 100ml of milk or other liquids.3 measuring cups (5g) every 100g of grated fruit.
INGREDIENTSMaltodextrine, Bioecolians® (glucooligosaccharide with prebiotic action), Inositol (Vitamin B7).
CHARACTERIS...
-
Product Destrolac confort
Food supplementation of milk in children with facility of regurgitation.
DOSAGEDissolve 5 g of powder equal to 3 measuring cups every 100 ml of water or milk.
INGREDIENTSCorn starch (52%), Maltodextrine, Vitamin B7 (Inositol).
CHARACTERISTICSDestrolac Confort is a Vitamin B...
-
Product Crossover Junior vials
Crossover Junior Royal Jelly and Carnitine is a dietary supplement specially developed for kids that practice also competitive sports activities and it is useful to compensate for an intense energy expenditure.
DOSAGE
The recommended daily dose is 1 vial a day preferably taken an hour befor...
-
Product Crossover Junior sachets
Crossover Junior Mineral Salts, Royal Jelly and Zinc is a dietary supplement specifically developed for kids that practice also competitive sports activities and is useful in the treatment of dehydration caused by excessive sweating combined with intense energy expenditure. Zinc contributes to the normal f...
-
Product Coredox drops
Dietary supplement with vitamin C and vitamin E with protective and antioxidant function against the free radicals.
DOSAGE10 drops per day unless otherwise medical advice.10 drops contain:Vitamin C = 50 mgVitamin E = 20 mg
INGREDIENTSVitamin C + Vitamin E (Ascorbic acid and Tocopherol...
-
Product Flogodren caps
Dietary supplement with effective anti-inflammatory and anti-edema action based Bromelain from Pineapple, Escin from Horse Chestnut and Boswellia serrata resin.
DOSAGE It is recommended to take 1 capsule per day.
INGREDIENTS
Bromelain, Escin from Horse Chestnut and Bos...
-
Product Biolan cream
For its affinity and its high hydrating and emollient power, it can be applied in the protection of the hurting nipples, in the emollient and lenitive treatment of the breast’s and anal’s rhagades.
DOSAGEBreast Rhagades: Soften a little quatity of the product with the cleaned fingers and ap...
-
Product Chetodral sachets
Chetodral sachets contrast effectively the states of ketosis and acetonaemic vomit of children and it is able to act in the complex metabolic alterations during the acidosis by acting constantly.
DOSAGE1 sachet dissolved in 100ml of water (a glass) 3 times per day.
INGREDIENTSMixed su...
-
Product Cobalavit drops
Adjuvant in the treatment of the hematological disorders or of the pernicious/megaloblastic anemia.
DOSAGE
2/25 per day.
1 drop contains: 1,25 mcg of Vitamin B12.
INGREDIENTS
Vitamin B12 (Cianocobalamina).
CHARACTERISTICS
Vitamin B12 in aqueous pha...
-
Product Grinsol Fitoaerosol
Fitoaerosol with marine water in isotonic solution. Medical device indicated as a specific adjuvant for typical cold symptoms such as nasal congestion and nasal mucus irritation.
Reduces the Symptoms of the Cold. It promotes Muco Fluidification. In case of allergic rhinitis and sinusitis.
...
-
Product Lioflor vaginal douche
Rejuvenating the bacterial flora - refreshing - antibacterial. CHARACTERISTICS Vaginal lavender with hyaluronate sodium, tea tree oil, calendula and active lactic ferments. FORMAT 5 100 ml Bottles with tank cap and 5 monouse cannula
-
Product Post-translational modifications
Intertek offers wide range of pharmaceutical services which includes post-translational modifications. It belongs to biopharmaceutical protein analysis services category. It includes deamidation, glycosylation, glycylation, phosphorylation, acetylation , alkylation, sulfation, etc.
-
Product Bifendatatum
Name:Bifendatatum or Biphenyldicarboxylate
CAS NO:73536--69-3
Chemical Name:
alpha-Diphenyldicarboxylate;Dimethyl 4,4'-dimethoxy-5,6,5',6'-dimethylenedioxybiphenyl-2,2'-dicarboxylate;7,7'-Dimethoxy-(4,4'-bi-1,3-benzodioxole)-5,5'-dicarboxylic
...
-
Product Alarelin
CAS No. : 79561-22-1.Molecular Formula: C56H78N16O12 Main product: Oxytocin Acetate,Vasopressin Acetate,Desmopressin Acetate,Terlipressin acetate, Caspofungin acetate, Micafungin sodium, Eptifibatide acetate, Bivalirudin TFA, Deslorelin Acetate,Glucagon Acetate,Histrelin Acetate,Liraglutide Acetate...
-
Product LYCADEX® BioPharma - Dextrose monohydrate, low endotoxin, USP, EP, JP
Delivering secure supply with the right partner. Trust is the most essential ingredient.
-
Product KLEPTOSE® HP & HPB BioPharma - Hydroxypropyl betacyclodextrin, low endotoxin, USP, EP
Suitable for use in biopharmaceutical manufacturing and as an excipient for injectable dosage forms.
-
Product NEOSORB® BioPharma - Sorbitol, low endotoxin, USP, EP, JP
Delivering secure supply with the right partner. Trust is the most essential ingredient.
-
Product Dextrose Anhydrous BioPharma - USP, EP, JP
Delivering secure supply with the right partner. Trust is the most essential ingredient.
-
Product 2-Bromo-N-Isopropylacetamide II Belumosudil II CAS : 75726-96-4
2-Bromo-N-Isopropylacetamide II Belumosudil II CAS : 75726-96-4
-
Product 4-Amino-2-Methyl-10H-Thieno [2, 3-B] [1, 5] Benzodiazepine Hydrochloride II Olanzapine Intermediate II CAS 138564-60-0
4-Amino-2-Methyl-10H-Thieno [2, 3-B] [1, 5] Benzodiazepine Hydrochloride II Olanzapine Intermediate II CAS 138564-60-0
-
Product N-(3-methoxyphenyl)piperazine II Letermovir Intermediate II CAS: 16015-71-7
N-(3-methoxyphenyl)piperazine II Letermovir Intermediate II CAS: 16015-71-7
-
Product Dopamine hcl
① Low blood pressure regulator ② Antishock agent ③ Raw material for the synthesis of norepinephrine
-
Product Protocatechuic acid
Synonyms: 3,4-Dihydroxybenzoic acid CAS: 99-50-3 Molecular Formula: C7H6O4 Molecular Weight: 154.12 Properties: White to slightly brown needle shaped crystals; soluble in hot water, ethanol, and ether, slightly soluble in water, insoluble in benzene and petroleum ether.
-
Product Single Use Products
We offer a very wide range of single-use products for the pharmaceutical and biopharmaceutical industries. We also offer customised solutions that can be easily adapted to your installations.
single use Assemblies, Back pressure regulators, pinch valves, plastic closure etc...

-
Product Pancreatin
Production from glands collected by own personnel at approved slaughterhuses. EU-GMP audit applied for 2018
-
Product Vaccine Services
Vaccine Drug Product and Finished Goods Services Development and manufacturing capabilities designed with patient safety and centricity in mind. We use robotic systems with single use product path to meet your program needs, providing pre-clinicalbatches up to 10,000 units and commercial batches up to 150,...
-
Product Drug Product & Finished Goods
End-to-endservices designedfor patient safetyand centricity while offering contracting convenience, cos tefficiencies and time savings. Our globally integrated drugproduct (DP) and finished goods (FG) services offer agileand flexible solutions for clinical to commercial products. Our DP capabilities includ...
-
Product Hyluronic Acid (Sodium Hyaluronate)
Hyaluronic Acid (Sodium Hyaluronate) is a well-established active pharmaceutical ingredient of non-animal origin. Manufactured by bacterial fermentation (streptococcus zooepidemicus), it is non-GMO and offers a wide range of application possibilities within • Orthopedics • Ophthalmology • Aesthetics • Viro...
-
Product Cationic Adjuvant Formulation CAF®
Croda Pharma continuously increases its offering in innovative vaccine adjuvant systems. The strategic collaboration with SSI (Statens Serum Institut, leading Danish governmental life-science research institute) enables us to offer an even more diverse adjuvant portfolio.
The CAF® t...
-
Product Aprotinin (bovine lung) powder
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our product list in the download files. For further information, please contact us: www.kraeber.de
Aprotinin is a natural polypeptide which originates from bovine lungs....
-
Product Chitosan Pharma Grades, low/medium/high molecular weight
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our product list in the download files. For more information, please contact: www.kraeber.de
-
Product Human Hepatitis B Immunoglobulin
The manufacturing process for human hepatitis B immunoglobulin contains a step of low pH incubation for viral inactivation. It contains a suitable amount of glycine as stabilizer, but does not contain any antiseptic or antibiotic.
Indications:
-Post-exposure prophylaxis should be c...
-
Product Human Albumin
The main component of this preparation is human albumin, which is prepared by cold ethanol fractionation of human plasma from healthy donors. The manufacturing process of human albumin contains a step of heating the final solution at 60℃ for 10 hours to inactivate the viruses present in it. It contains...
-
Product Human Lmmunoglobulin
The main component of this preparation is human immunoglobulin, which is prepared by cold ethanol fractionation of human plasma from healthy donors. The manufacturing process contains a step of low pH incubation for viral inactivation. It contains a suitable amount of glycine as stabilizer, but does n...
-
Product Human Tetanus Immunoglobulin
The main component of this preparation is human tetanus immunoglobulin, which is prepared by cold ethanol fractionation of human plasma from donors immunized against tetanus. Donations are selected on the basis that they contain high titers of antibodies against tetanus. The manufacturing process for h...
-
Product Human Rabies Lmmunoglobulin
Human rabies immunoglobulin is prepared by cold ethanol fractionation of human plasma from donors immunized against rabies. The manufacturing process for human rabies immunoglobulin contains a low pH incubation step for viral inactivation. It contains a proper amount of glycine as stabilizer, but does ...
-
Product Human Immunoglobulin (Ph4) For Intravenous Injection
The main component of this preparation is human immunoglobulin, which is prepared by cold ethanol fractionation of human plasma from healthy donors. The manufacturing process contains a step to remove anticomplementary activity and a dual viral inactivation process.
【Indications】
q...
-
Product Bovine serum albumin, fraction v
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Bovine serum, sterile filtered
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Bovine serum, prefiltered
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Bovine serum, non sterile
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Bovine serum, 0,1µm sterile filtered
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Chondroitine sulfate a+c
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Chicken serum, non sterile
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product L-erythrulose
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product N-acetyl-d-glucosamine
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product D-glucosamine sulfate potassium chloride
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product D-glucosamine sulfate sodium chloride
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Choriongonadotrophin, powder
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Bovine plasma, non sterile
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Bovine plasmasubstrate r
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Bovine serum, prefiltered, heat-inactivated
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Bovine serum, sterile filtered, gamma-irradiated
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Chitosan carboxymethyl
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Chicken plasma, non sterile
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Chicken plasma, sterile filtered
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Chitin
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Chitosan hcl
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Chitosan lactate
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Chitosan oligomer
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Chitosan standard diverse, various degree of deacetylations and viscosities
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For more information, please contact us: www.kraeber.de
-
Product Glucosamin / chondroitin sulfate blends
Kraeber & Co GmbH offers wide range of pharmaceutical products which includes glucosamin / chondroitin sulfate blends. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product MSM - Methyl Sulfonyl Methan
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Heparin Sodium
Kraeber & Co GmbH offers a wide range of pharmaceutical products. We are GMP, GDP and ISO 9001:2015 certified. You can find our catalogue and product list in the download files. For further information, please contact us: www.kraeber.de
-
Product Heparin calcium
From mucosa to API-all at one production site. EU-GMP audit applied for 2018
-
Product Enoxaparin Sodium
Production starts from our own Heparin Sodium.. EU-GMP inspection applied for 2018.
-
Product Enoxaparin calcium
Production starting from our own Heparin Sodium.. From mucosa to API - all at one production site.
-
Product Lung Surfactant
At development stage. From pig lungs making use of our controlled collection with own personel in approved slaughterhouses.
-
Product Liver Extract
Yino pharma offers a wide range of products which includes Liver Extract. Contact us for more information.
Biopharmaceuticals
Biopharmaceuticals, also known as biologic medical products, are drug products manufactured using biotechnology. Biopharmaceutical drugs are structurally similar to various compounds within the body. Over time, drug development has progressed from small-molecule compounds to large molecule proteins and other accompanying biopharmaceuticals. The discovery of biopharmaceuticals has significantly improved the healthcare delivery. It has provided a shift in providing cures for chronic illnesses such as refractory cancers, diabetes, autoimmune diseases, etc., thereby improving the affected patients' quality of life.
The use of biopharmaceuticals for therapeutic purposes dates as far back as the 19th century, with recombinant human insulin being the first approved biopharmaceutical used for therapeutic purposes in human beings. Over the last three decades, the biopharmaceutical industry has experienced notable growth and is expected to contribute to some remarkable pharmaceutical industry developments. Biopharmaceutical offers a wide range of advantages and benefits to pharmaceutics, and here, we would be exploring these benefits. We would also examine the biopharmaceutical manufacturing process, notable examples of biopharmaceuticals, and more.
What are biopharmaceuticals?
These complex medicines are proteins (monoclonal antibody), sugars (glycan), nucleic acids (RNA, DNA), living cells, or tissues extracted or semi synthesized from a biologic source by biotechnology for in vivo diagnosis or therapeutic purposes.
The majority of biopharmaceuticals are synthesized or derived from living organisms or genetically modified cells and organisms. They cannot only lessen symptoms or treat chronic diseases but also cure them with little or no side effects because of their origin and specificity. Some biopharmaceuticals include enzymes, antisense drugs, cytokines, monoclonal antibodies, gene therapy, cell therapy, etc.
Biopharmaceuticals have helped personalize the treatment process to meet the specific health needs of patients. This is achieved through the modification of drugs genetically through DNA-recombinant technologies or cell fusion to alter specific factors to cure, prevent, or diagnose individual diseases.
How are biopharmaceuticals made, and why?
As opposed to chemical synthesis through which conventional medicines and drug products are made, biopharmaceutical drugs are synthesized through more complex processes from living cells. With the rapid growth of the biopharmaceutical industry and the discovery of new biologic therapies, the term biopharmaceutical (biologics) refers to a variety of medicines and drug products. The diversified portfolio contains various drugs produced by different biotechnological methods and techniques.
These drug products are not made from chemical mixtures but rather from living organisms. Experts in cellular biology, biochemistry, and several other fields carry out biologic testing and analysis (using analytical methods) to ensure that the desired end product is made.
For example, biologics used to treat joint pain are made by adding a DNA into a living cell such as a yeast or mammalian cell to enable the production of a specific molecule in large amounts. These molecules are usually therapeutic proteins that are isolated and utilized as active ingredients.
When newly introduced, biopharmaceuticals focused solely on developing recombinant protein such as enzymes (TPA) and hormones (insulin, erythropoietin, growth hormone, etc.) through recombinant DNA techniques. With time, they have delved into the design and development of chemically defined cells, manufacturing of monoclonal antibodies, and genome-based technology. These new techniques have improved the pharmaceutical manufacturing process and created new treatment options for different diseases.
Like every other drug, biopharmaceutical drugs are produced for therapeutic purposes, but with a catch. They are produced majorly to cure chronic and dilapidating diseases such as cancer, diabetes, rheumatoid arthritis, and several others. The manufacturing of these drug products also aims at providing effective, safe, and cost-effective drugs for therapeutic purposes.
What is the biopharmaceutical industry?
The biopharmaceutical industry is an essential subset of the pharmaceutical industry. It is comprised of biopharmaceutical companies that discover, develop, produce, and market biopharmaceuticals. The drug products made are used to diagnose, treat, prevent, and cure diseases when administered to patients. This industry deals majorly in biopharma drugs and medicines produced via the biotechnological process from living entities such as micro-organisms, living cells, tissues, or organs of humans and animals. The biopharmaceutical industry successfully merges between biotech and pharmaceutical companies to provide better and safer healthcare to patients.
What is the difference between biopharmaceutic and biopharmaceuticals?
Biopharmaceutics is simply pharmaceutics that deals with biopharmaceuticals. It is a branch of pharmaceutical science that examines the physiochemical properties of drugs, their toxicology, pharmacology, dosage forms, and patients' clinical response to drugs, biopharmaceuticals inclusive. The recommended dosage and dosing interval varies for different drugs, and it significantly affects their efficacy and safety. Biopharmaceutics provide adequate information and understanding of the pharmacodynamics and pharmacokinetics of biopharmaceutical drugs, their metabolites, and their effects on the body. Through biopharmaceutics, biopharmaceutical drugs can be evaluated based on their properties and effects on the body.
On the other hand, biopharmaceuticals refer to inherently biologic pharmaceuticals extracted, synthesized, or semi-synthesized from live forms using biotechnological methods. They constitute a significant part of the drug products examined and evaluated in biopharmaceutics to improve healthcare delivery and, in turn, the quality of patients' lives.
How are biopharmaceuticals manufactured?
The rapid advancement of genetics and molecular biology has played a significant role in the biopharmaceutical manufacturing process. The introduction of new and refined techniques such as protein purification, host system, etc., has positively impacted the efficacy, capacity, and safety of biopharmaceuticals produced.
There are many biopharmaceutical manufacturing processes, and they vary based on the type of drug product to be produced.
Where is biopharmaceutical manufacturing heading?
With several emerging technologies in the biopharmaceutical manufacturing process, the biopharmaceutical manufacturing process is expected to take a huge turn. With over 1000 biopharmaceuticals in clinical trials, the majority of drugs in the future may be biopharmaceuticals. The increased need for biopharmaceuticals and the constant advancement in biotech will impact biopharmaceutical manufacturing. Given the various advantages of gene therapy, biopharmaceuticals' manufacturing process is likely to focus more on gene therapy in the future.
What is the process involved in the process of manufacturing biopharmaceuticals?
The process of small-scale and large-scale biopharmaceutical production varies. However, biopharma companies produce to indulge in large-scale biopharmaceutical production. The processes involved in manufacturing these drugs are complex, lengthy, and largely differ depending on the end-product. Generally, the processes include biosynthesis, purification, formulation, and final dosage preparations.
Materials and equipment are sterilized at the biosynthetic stage, and a cell culture or biologic extract is harvested or collected in a bioreactor to grow and multiply. During the purification process, cells, tissues, enzymes, or desired products are separated from surrounding nutrients and byproducts to produce the bulk product. Unlike conventional drugs mixed with other chemical components during formulation, biopharmaceuticals are made and sold as sterile products. Although they are sold as sterile products, biopharmaceuticals are packaged in similar ways as conventional drugs before distribution and sales.
This description gives a general knowledge of the manufacturing process carried out. Nevertheless, there are special intricate biotech processes that can be carried out and some include continuous manufacturing, and other processes requiring single use technology.
What special considerations must be made when manufacturing biopharmaceuticals?
Consistent safety, effectiveness, and quality are the essential factors that must be considered during the biopharmaceutical manufacturing process.
The manufacturing processes of each biopharmaceutical product must be designed to produce consistent quality and safe biopharmaceutical drug products. This involves the complete removal of contaminants and impurities such as viral contamination, endotoxins, cell membranes, and others from the separated product. Also, regular rigorous and well-documented checks and examinations on the efficiency and performance of the materials and equipment must be carried out to validate the manufacturing process.
Benefits of biopharmaceuticals
Given the structural composition and origin of biopharmaceuticals, these bio drugs provide several health benefits to patients and, in extension, the healthcare industry and healthcare professionals. The emergence of biopharmaceuticals has provided patients with a better shot at quality life and longevity.
How do patients benefit from biopharmaceuticals?
Biopharmaceutical drugs, unlike conventional medicines, mimic the structures of human compounds, and this gives biopharmaceuticals the potential and ability to cure diseases more effectively. They also produce fewer side effects due to their high specificity, unlike the traditional medications that are capable of affecting multiple systems simultaneously. Given their similarities with human compounds, they are very safe and highly effective.
Also, these drugs can be genetically modified to allow healthcare professionals to tailor treatments to meet patients' specific health needs. In addition, some generic drugs play crucial roles in providing patients with curative and diagnostic benefits.
However, one of the greatest potentials of biopharmaceuticals that also serves as an essential benefit to patients is gene therapy. Through gene therapy, conditions characterized by rapidly dividing cells due to mutated or defective genes, such as cancer, can be cured. Unlike some cancer drugs and other medications also, biopharmaceutical drugs are non-carcinogenic. The innovation of biopharmaceuticals also aids in producing varieties of vaccine to prevent infection and the spread of infectious diseases in patients.
Generally, bio drugs improve longevity, increase life expectancy, provide welfare to the population, and provides several public health benefits.
How does the healthcare industry benefit from biopharmaceuticals?
The impact of biopharmaceuticals and biopharmaceutical innovation on health care professionals also tells on the healthcare industry. Biopharmaceuticals have a massive positive economic impact, and other healthcare industry benefits that cannot be ignored. The development and availability of the various biopharmaceuticals in the pharmaceutical market will increase access to bio drugs, cost-effective medicines, and a boost in the global pharmaceutical market. Also, the ease of commercial production of biopharmaceuticals boosts the healthcare industry's economy and market growth.
How do healthcare professionals benefit from biopharmaceuticals?
Biopharmaceuticals and biopharmaceutical innovations provide outstanding benefits to healthcare professionals. It provides healthcare professionals with a variety of safe and efficient curative and therapeutic options for their patients. Therefore, in cases where a particular bio drug doesn't produce the desired therapeutic effect in a specific patient or has undesired side effects, other alternatives within the same drug family can be administered. Also, biopharmaceutical innovation provides job opportunities for healthcare professionals and individuals in different fields of practice.
Examples of common biopharmaceuticals
There are various biopharmaceutical products, and the majority of these products are widely known. Some common biopharma products include insulin, blood factor IX and VIII, human growth hormone, erythropoietin, tissue plasminogen activator, therapeutic antibodies, glucocerebrosidase, interferon α, and β, and GSCF. These form the most common examples, but they fall into the different biopharmaceutical classes, including hormones, clotting factors, blood products, vaccines, cytokines, and growth factors.
Where can some common biopharmaceuticals be found?
Biopharmaceuticals are derived and produced from various cells. They can be extracted and synthesized mammalian cell lines, microbial cells (yeast culture or recombinant E. coli), moss plants, and plant cell cultures. The majority of these sources are readily available and accessible at all times. These sources are origins from which the drug development process spans.
Who commonly uses biopharmaceuticals?
Biopharmaceuticals are primarily used for patients. However, most of the patient population that uses biopharmaceuticals are patients with long-term diseases such as psoriasis, diabetes, Crohn’s disease, rheumatoid arthritis, etc. The elderly constitute the majority of patients affected by either of these chronic conditions. Also, cancer patients form a significant percentage of the patient population using biopharmaceutical products.
What industries can common biopharmaceuticals be found in?
The common biopharmaceuticals are produced majorly by the biopharmaceutical and biotechnology industries. Within these industries, these biopharmaceutical products are developed and manufactured by biopharmaceutical companies. Some of the top pharma companies that manufacture the most common biopharma products include Gilead Sciences, Amgen, Genentech, Biogen, Riotech, Samsung biologics, Teva Pharmaceuticals, Celgene, Pfizer, United Therapeutics Corporation, UCB, and Sanofi. Although these companies form the existing biopharmaceutical manufacturing companies, a new biopharmaceutical company, Clover biopharmaceuticals, presently undergoing clinical trial is set to become a top tier pharmaceutical company in this industry.
References
https://medcraveonline.com/OAJS/biopharmaceutical-innovation-benefits-and-challenges.html
References
Upcoming Events
-
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025 -
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025 -
CPHI & PMEC China 2025
Shanghai New International Expo Center
24 Jun 2025 - 26 Jun 2025
Pharmaceutical Industry Webinars
-
Webinar An Untapped Market in Gender Inclusivity and Equity
-
19th March 2025
-
3pm GMT /4pm CET
-
-
Webinar Pathway to $10/g Biologics Production: Reshaping the Biomanufacturing Landscape
-
18th February 2025
-
3pm BST /4pm CET
-
-
Webinar Leveraging Real-Time Clinical Data to Deliver Certainty in Solubility Enhancement and Modified Release Development
-
12th December 2024
-
3pm BST/ 4pm CET
-
-
Webinar The CPHI Sustainability Collective: A New Initiative to Support a Sustainable Pharma Value Chain
-
10th December 2024
-
3pm BST /4pm CET
-
-
Webinar Key to Success: A CDMO's Pathway to Biologics Excellence
-
5th November 2024
-
3pm BST/ 4pm CET
-
-
Webinar The Changing Dynamics of Global API Manufacturing
-
17th September 2024
-
3pm BST/ 4pm CET
-
-
Webinar Shaping the Future of Italy’s Pharma Market: Trends and Opportunities
-
4th September 2024
-
4pm CET/ 10am EST
-
-
Webinar Fragment-Based Oligonucleotide and Oligopeptide Synthesis
-
30th Jul 2023
-
4pm CET / 10am EST
-
-
Webinar GMP Rationale for Sterile High-Potency/Toxic Pharmaceuticals
-
18th June 2024
-
4pm CET / 10am EST
-
-
Webinar Unlocking Opportunities in the Growing Pharma Landscape of The Middle East
-
5th June 2024
-
3pm CET / 9am EST
-
-
Webinar Exploring Technological Trends in the Future of Pharmaceutical Manufacturing
-
23rd May 2024
-
4pm CET / 10am EST
-
-
Webinar Achieving Manufacturing Excellence Through Digital Transformation
-
16th April 2024
-
4pm CET / 10am EST
-
-
Webinar Made in Africa: What’s Driving Pharma Manufacturing
-
28th March 2024
-
4pm CET / 10am EST
-
-
Webinar Case Study: Risk Management for Annex 1 Sterile Production EMS
-
28th February 2024
-
4pm CET / 10am EST
-
-
Webinar Innovative Strategies for B2B Pharma Marketeers: Driving Value through Content
-
20th February 2024
-
4pm CET / 10am EST
-
-
Webinar Revolutionizing Pharma: Data and AI Unleashed
-
18th January 2024
-
4pm CET / 10am EST
-
-
Webinar Optimal Temperature: Elevating Biologics Cold Chain Excellence
-
16th January 2024
-
4pm CET / 10am EST
-
-
Webinar Market Outlook – The Biggest Pharma Trends of 2024
-
12th December 2023
-
4pm CET / 10am EST
-
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance
















































































































































.png)
.png)
.png)
.png)
.png)
.png)













.jpg)












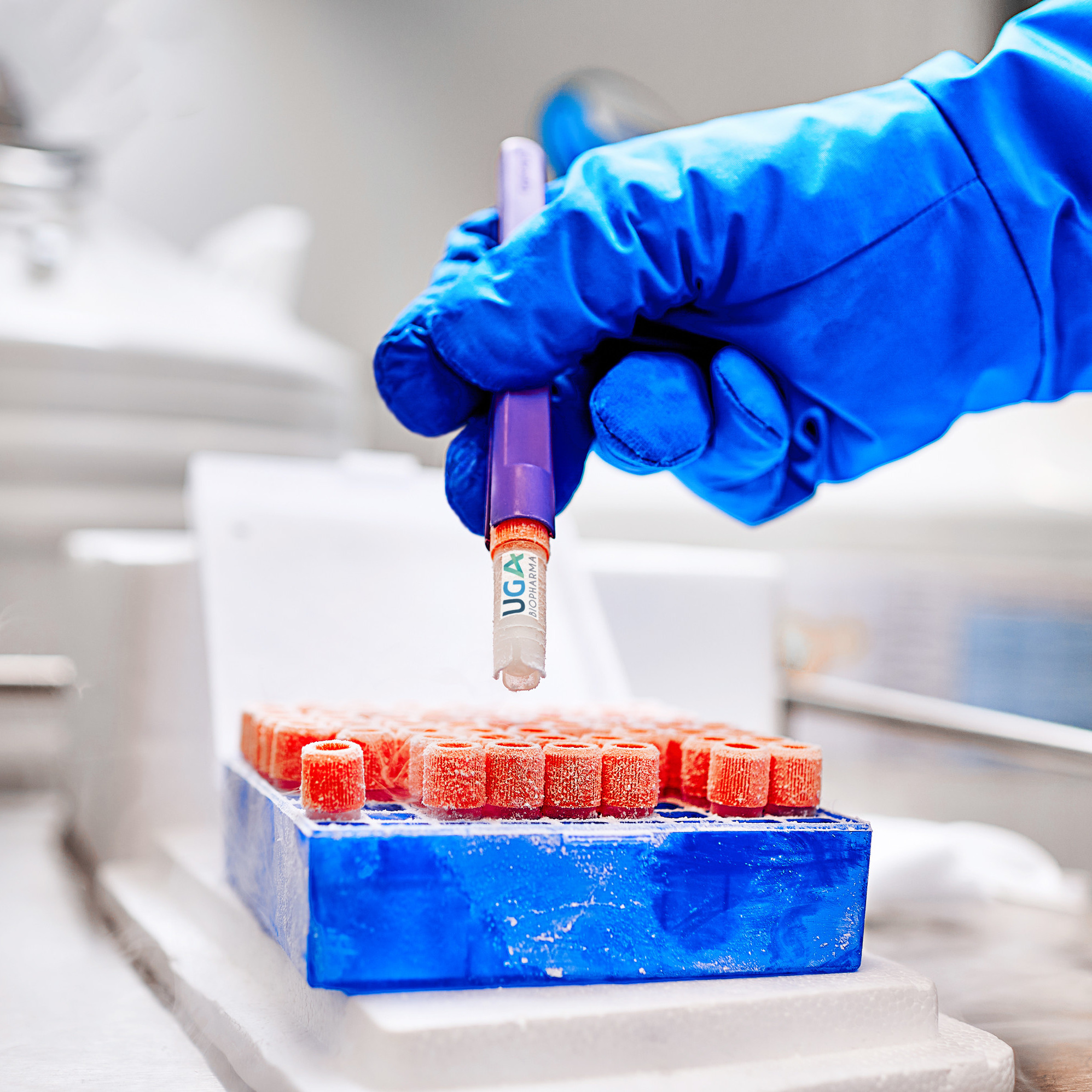














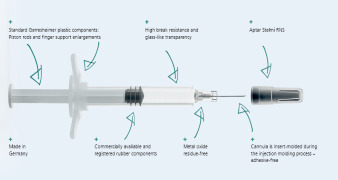






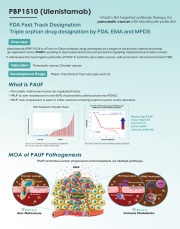





























.jpg)






























.png)



.png)







.png)

.png)



.jpg)
