Polpharma Biologics
About Polpharma Biologics
Certifications
Categories

-
PL
-
2021On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
Meet us at
CPHI Frankfurt 2025
Messe, Frankfurt
28 Oct 2025 - 30 Oct 2025
Products from Polpharma Biologics (1)
-
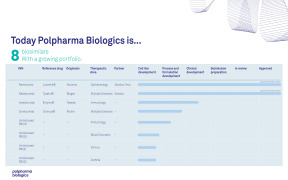
Product Pipeline
Polpharma Biologics has its own pipeline of biosimilars for out-licensing.
We are focused on advancing and expanding our product portfolio to increase patient access to these much-needed therapies and are open to partnering with other organizations to co-develop or commercialize our biosimilars.
Polpharma Biologics Resources (4)
-
News Polpharma Biologics’ investigational biosimilar shows PK/PD comparability to inflammatory bowel disease blockbuster Entyvio®
Polpharma Biologics, announced topline results demonstrating the pharmacokinetic (PK) and pharmacodynamic (PD) comparability of its biosimilar candidate PB016 to its reference drug, Entyvio®* (vedolizumab).
-
News Polpharma Biologics announces approval of Europe’s first and only biosimilar for multiple sclerosis - Tyruko® (natalizumab)
Polpharma Biologics announced that the European Commission (EC) has approved Tyruko® (natalizumab) as the first and only biosimilar for relapsing forms of multiple sclerosis (MS) in Europe. Tyruko® was developed by Polpharma Biologics and will be commercialized by its collaboration partner Sandoz.
-
News Polpharma Biologics announces FDA approval of Tyruko®
Polpharma Biologics, announced that the FDA is the first regulatory body worldwide to approve the use of Tyruko® (natalizumab-sztn) - a new biosimilar for the treatment of relapsing forms of multiple sclerosis (MS). Approval of Tyruko® by the European Medicines Agency is also expected imminently.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance









.jpeg)