- Home
- CuraTeQ Biologics
- Biosimilars Portfolio
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
Biosimilars Portfolio
Product Description
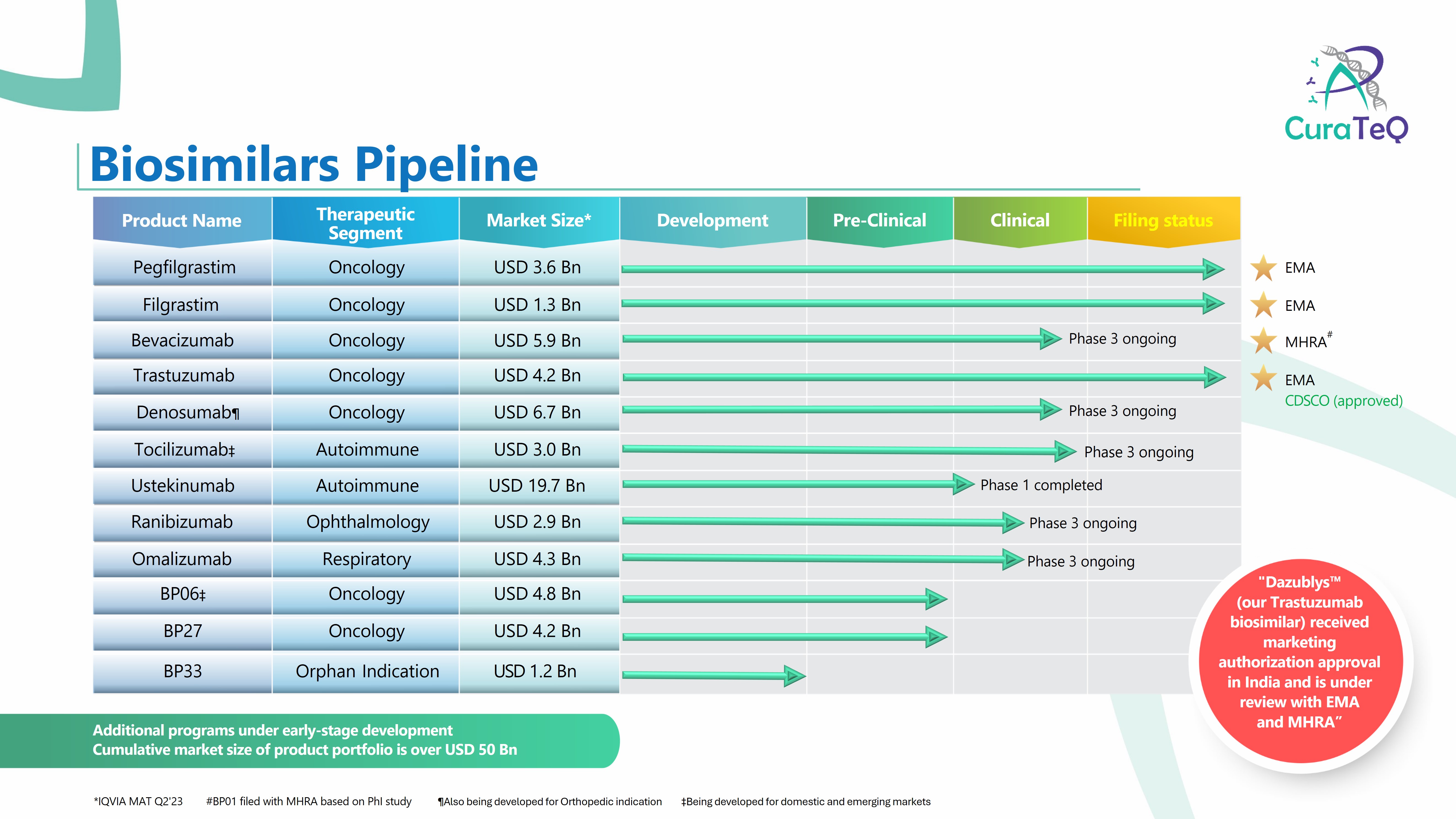
- Pegfilgrastim (Filed with EMA)
- Filgrastim (Filed with EMA)
- Trastuzumab (Filed with EMA, MHRA, Received recommendation for marketing authorization in India)
- Bevacizumab (Filed with MHRA)
- Omalizumab (Global PhIII ongoing)
- Denosumab (Global PhIII ongoing)
- Ranibizumab (Global PhIII ongoing)
- Tocilizumab (PhIII ongoing)
- Ustekinumab (PhI complete)
CuraTeQ Biologics

-
IN
-
2024On CPHI since
-
250 - 499Employees
Company types
Biopharmaceutical company
CMO/CDMO
Generics/Biosimilars Manufacturer
Pharmaceutical company
Primary activities
Biopharmaceutical
Contract Manufacturer
Custom Manufacturing/Custom Synthesis
Generic APIs producer
Pharmaceutical Company (generic finished products)
Contact info
-
-
Survey No. 77 & 78, Indrakaran Village,, Kandi Mandal, Sangareddy District, 502329, Hyderabad, Telangana, India
Departments
Business Development
-
 Pradyumna MulpurDeputy General Manager - Program Management & Business DevelopmentHyderabad, Telangana, India
Pradyumna MulpurDeputy General Manager - Program Management & Business DevelopmentHyderabad, Telangana, India -

Other
-
Satakarni MakkapatiChief Executive Officer and Board MemberHyderabad, India
-
-
Dr. Venkata Ramireddy YeturuSenior Vice President - Program Management and Business DevelopmentHyderabad, Telangana, India
Categories
- Biopharmaceutical Products Biomanufacturing
- Biopharmaceutical Products Biopharmaceuticals (General Category)
- Biopharmaceutical Products Biosimilars
- Biopharmaceutical Products Monoclonal Antibodies
- Contract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services)
- Generic APIs Generic Antibodies
- Innovative APIs Antibodies
- Packaging Materials and Equipment (Products) Injectable systems and components
Sales Markets
CuraTeQ Biologics

-
IN
-
2024On CPHI since
-
250 - 499Employees
Company types
Biopharmaceutical company
CMO/CDMO
Generics/Biosimilars Manufacturer
Pharmaceutical company
Primary activities
Biopharmaceutical
Contract Manufacturer
Custom Manufacturing/Custom Synthesis
Generic APIs producer
Pharmaceutical Company (generic finished products)
Contact info
-
-
Survey No. 77 & 78, Indrakaran Village,, Kandi Mandal, Sangareddy District, 502329, Hyderabad, Telangana, India
Departments
Business Development
-
 Pradyumna MulpurDeputy General Manager - Program Management & Business DevelopmentHyderabad, Telangana, India
Pradyumna MulpurDeputy General Manager - Program Management & Business DevelopmentHyderabad, Telangana, India -

Other
-
Satakarni MakkapatiChief Executive Officer and Board MemberHyderabad, India
-
-
Dr. Venkata Ramireddy YeturuSenior Vice President - Program Management and Business DevelopmentHyderabad, Telangana, India
More Products from CuraTeQ Biologics (1)
-
Product Synthetic Peptide APIs Portfolio
US DMF Approved: • Vasopressin • Icatibant acetate • Leuprolide acetate • Linaclotide • Etelcalcetide • Desmopressin US DMF Filed: • Calcitonin salmon • Ganirelix acetate • Degarelix • Glatiramer acetate • Plecanatide • Teriparat...
CuraTeQ Biologics resources (2)
-
Brochure Biosimilars Pipeline
CuraTeQ Biologics is a biopharmaceutical company based in Hyderabad, India. We have a pipeline of 14 biosimilars of which: 3 products are filed with EMA, one product is filed with MHRA, and 4 products are in global Ph3 clinical trials. Dazublys™, our Trastuzumab biosimilar has received marketing authorization approval in India, and is also filed with EMA. -
Brochure Synthetic Peptide APIs Portfolio
Auro Peptides, our synthetic peptides arm, has filed 14 US DMFs which have contributed to 6 ANDA approvals.
Frequently Viewed Together
-
Product UV Light Blocking Tyvek Equipment Covers
UV Light Blocking Tyvek Equipment Covers are designed to keep cleaned pharmaceutical equipment and supplies clean and protected from external sources of contamination while also providing in-process protection to light sensitive biotech products.
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product RoSS® Shell: Protection of single-use bags
Protect your single-use bioprocess containers: RoSS® Shells - abbreviated for Robust Storage and Shipping - reduce product loss towards 0%. Compatible with all available sizes and types of single-use bags, RoSS enables standardized and scalable end-to-end process solutions for fluid management and col...
-
Product IDT Biologika as a Contract Development and Manufacturing Organization
IDT Biologika is an international leader in the contract development and manufacturing of biologics. The company focuses on the customized development and manufacturing of viral vaccines (phases I, II, III), gene therapeutics, immunotherapeutics, oncolytic viruses as well as sterile liquid and lyophilized ...
-
Product Analytical Services
Actylis performs routine and non-routine analytical testing for various industries, in support of manufacturing processes, QA/QC functions, R&D projects, and environmental applications. Leveraging state-of-the-art instrumentation, extensive knowledge base, as well as internal, cross-functional competen...
-
Product Pharmaceutical and Biological Products Contract Development and Manufact...
Bora Pharmaceuticals is a premier international contract development and manufacturing organization (CDMO). Our six state-of-the-art cGMP manufacturing facilities across Asia and North America deliver to more than 100 markets around the world. Our sites have the highest industry standards for quality, dev...
-
Product Drug-device combinations
Deep technical expertise spanning device conceptualization, drug-device evaluation, assembly process, cGMP supplies and testing
Capability to assemble and test multiple pens /autoinjector devices from different device manufacturers, offering great flexibility
Manufacturing excellence wit...
-
Product Peptide and Protein Technology
Almac’s peptide and protein technology offering is a key component within our suite of services. We offer a complete range of peptide and protein services from catalogue products through to GMP manufacture from early phase to commercial launch. We have a proven track record in high quality supp...
-
Product BioHale® Trehalose Dihydrate
BioHale® Trehalose Dihydrate is a non-reducing disaccharide consisting of two glucose molecules which are linked by an α, α-1,1 bond. It is used in the pharmaceutical industry as a stabilizer of biologics.
Trehalose Dihydrate has proven to be highly effective in protecting cell membranes...
-
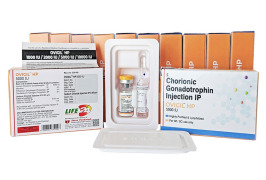
Product HCG/Chorionic Gonadotrophin for Injection -OVIGIL HP 5,000 IU
The Gonadotropin That Delivers – Precision, Reliability & Consistency
This product is available as a combi pack of Chorionic Gonadotrophin for Injection BP 5000 IU & Sodium Chloride Injection BP 0.9% w/v (HCG Injection).
• Use: This highly purified Human Chorionic Gonadotropin...
-
Product Vials
Mefar ilac sanayii offers wide range of products including vial filling between 2 ml through 300 ml.
Mefar's all services have EMA certification.
Contact us for more information.
-
Product Components for injection systems
As component of drug pumps, the drug channel – or fluidic outlet – serves an important interface function: It transports the liquid pharmaceutical from the reservoir into the human body.
• Drug-compatible, coextruded micro-tubing with inert inner layer • Reduced pain for the patient thanks to s...
-
Product Walk in Cooling Cabinet.
The Walk-In Cooling Cabinets / cold rooms are designed according to ICH guidelines, WHO, MCA and USFDA requirements to maintain uniform conditions. They are superior in airflow distribution, temperature control technology, cabinet construction and are manufactured as per cGMP regulations.
Biggest Walk...
-
Product Mineral Phosphates
Dr. Paul Lohmann(R) offers a wide range of Mineral Phosphates for the Biopharmaceutical industry. They are widely used in various processes such as upstream, downstream and drug formulation and are an essential part of the entire biomanufacturing process. We also offer buffers as solid premixes or readily ... -
Product CordenPharma Oligonucleotide Platform
Your Expert Partner for Oligonucleotide APIs
• Process Development & Small-scale: scale-up to 10 mmol per run(small-scale equipment can also support early-stage supply)
• Scale-up & Small to Medium-scale: scale-up to 100 mmol per run(supported by OligoPilotTM equipment)
• ...
-
Product Pharma Separators
GEA pharma separator skids aseptic and pure are energizing the industry with two comprehensive and sophisticated separator lines and one revolutionary new multi-bowl solution. These plug & produce pharma separator skids are breaking new ground in ensuring maximum safety, maximum yield and perfect adaptatio...
-
Product Lipid nanoparticles
Lipid nanoparticles (LNPs) enable the delivery of a variety of molecules, including nucleic acids such as mRNA, to cells and are therefore an essential tool in gene therapy. To unleash the potential of vaccines, protein and gene therapies, drug developers need the support of a trusted and experienced pro...
-
Product SAN-DV Pro
For use in combination with drugs in vials, the SAN-DV Pro was designed as a fully disposable automatic needle with an integrated vial adaptor for easy drug reconstitution or mixing.
The SAN-DV Pro reduces patient anxiety and is easy to use. Additional...
-
Product Fermentation
Relying on an experience gained over more than 50 years, OLON represent one of the most extensive know how of microbial fermentation in Europe. The Group, global leader in biomanufacturing, has two Biotechnology Centres located in Italy and is one of the first companies in Italy producing via microbial ...-comp246698.jpg)
-
Product Post-translational modifications
Intertek offers wide range of pharmaceutical services which includes post-translational modifications. It belongs to biopharmaceutical protein analysis services category. It includes deamidation, glycosylation, glycylation, phosphorylation, acetylation , alkylation, sulfation, etc.
-
Product Chorionic Gonadotrophin (HCG)
DADELI is a GMP-certified pharmaceutical manufacturer for intermediates,APIs and finished formulations.Our main APIs include HCG, Aprotinin(Currently in China we are the only one to produce Aprotinin complying with the latest EP 11.0), Oxymatrine,Isoniazidum,Egg Lecithin,Daidzein,etc.Warmly welcome all cus... -
Product Steriles
We are your expert in sterile and aseptic manufacturing, where quality is paramount.
Your most trusted partner for sterile and aseptic fill & finish:
- Extensive knowledge and experience in process and analytical development
- Unbeatable speed to market, without compromising on quality
- ...
-
Product Cell Culture Services
FUJIFILM Diosynth Biotechnologies is a leading provider of cell culture services for biologics, advancing anddelivering life changing therapies. We offer complete solutions for cell line development, process development, late phase activities, clinical and commercial manufacturing of a wide variety of biop...
-
Product Sterile Grade at ISO Class 5 facility
Kikkoman can offer "sterile grade” products, purified through a super purification process at our ISO Class 5 facility, approved by the Japanese Authority.
-
Product CDMO Biopharmaceutical Production
With an extensive offering of development services, state-of-the-art manufacturing equipment, and project support, GBI is truly a full-service CDMO biologics partner. Our approach pairs our expert knowledge base with an open mind towards scientific discovery so that together we can develop an optimal way t...
-
Product Mipeginterferon alfa-2b
Pegylated human interferon alfa-2b
Trade name: PEGBING
Technology
Recombinant gene technology, expressed by Yeast
Amino acid
165 amino acids
Molecular weight
Approximately 59 kD
Indications
Chronic hepatitis B & C
...
-
Product Supply Chain Management
• GMP Compliant Storage of a wide range of client products from API and excipients to consumables as well as both Stability and Retention sample programs. • Facility and Staff certified for storage, handling, and shipment of hazardous materials. Including Flammable, Toxic, Potent, etc. products. • Envir...
-
Product Ergon vials
Ergon vials is a dietary supplement of Iron, Folic Acid, Vitamin B12 and Vitamin C, useful to fill nutritional deficiencies or increased needs of these nutrients. Iron contributes to normal transport of oxygen in the body, folic acid and Vitamin B12 contributes to the normal formation of red blood cells ...
-
Product Drug substance production
With our two single-use individual production suites, we double our capacity to meet the most demanding needs of our clients. Our modern facilities accommodate production volumes of 500L and 2000L, ensuring maximum flexibility for your biopharmaceutical projects.
Key products supported: ...
-
Product HANQUYOU(trastuzumab)
HANQUYOU (trastuzumab, trade name: HERCESSI™ in the U.S., Zercepac® in Europe) was successfully launched in the U.S., China and Europe, becoming the Chinese mAb biosimilar entering the U.S., the EU and China market. It is indicated for the treatment of HER2 positive early breast cancer, metastatic bre...
-
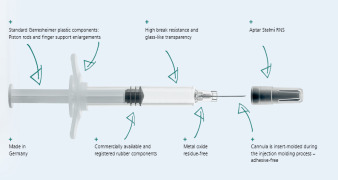
Product Gx RTF® ClearJect® Polymer syringes
Gx RTF® ClearJect® syringes are made from COP (Cyclo Olefin Polymer). They are glue-free as the cannula is not glued in, but is instead insert-molded during the injection molding process, no aggregation due to metal or tungsten residues, high viscosity silicone oil for reduced particles, minimal extractabl...
-
Product Biotechnology Services
Midas Pharma offers a wide range of pharmaceutical services which include biotechnology services. It offers: • development of biosimilars and proprietary biologicals (starting from gene sequence) • cGMP production (microbial and mammalian cells) • related bioanalytics (from protein biochemical meth...
-
Product Bispecific Antibody Production
Bispecific antibodies represent a key component of the next-generation of antibody therapy. Bispecific antibodies can target more than two different antigens at the same time. For instance, they can simultaneously bind tumor cell receptors while recruiting cytotoxic immune cells.Biointron has expressed tho...
-
Product Processing Vessels
Briggs have an extensive history in designing and manufacturing processing vessels for the pharmaceutical sector. We have experience in design and build to ASME or PED standards and we can offer vessels for any size of project from laboratory scale tank farms for large scale manufacturing. Our solutions ca...
-
Product DWRX1010 (Colonoscopy Bowel Preparation Drug)
※ DWRXs Differentiation Points 1) Maximize compliance in the form of minitablets. 2) Containing simethicone to remove foam in the colon, enabling smooth colonoscopy. 3) Maximizes the convenience of taking medication, and low water intake. ※Development Strategy 1) Non-clinical trial (KR) and patent applica...
-
Product Hyatrue® — Pharmaceutical Grade Sodium Hyaluronate
Hyatrue® is a pharmaceutical grade sodium hyaluronate which can be used as an API or excipient for drugs and medical devices in ophthalmic preparations, intra-articular injections, anti-adhesive preparations, topical preparations for wound healing and soft tissue filler, etc. Hyatrue® sodium hyaluronate ...
-
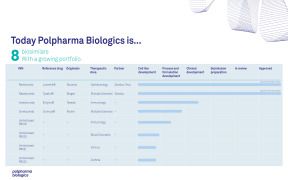
Product Pipeline
Polpharma Biologics has its own pipeline of biosimilars for out-licensing.
We are focused on advancing and expanding our product portfolio to increase patient access to these much-needed therapies and are open to partnering with other organizations to co-develop or commercialize our biosimilars.
-
Product Elizaria® (eculizumab)
Paroxysmal nocturnal hemoglobinuria + atypical hemolytic uremic syndrome

-
Product Walk-In/ Drive In Solutions
FARRAR® leverages deep-application expertise and a broad range of equipment and services to deliver +40°C to -80°C walk-in/drive-in systems that meet your precision-temperature needs and sustainability goals. We understand the challenges involved in maintaining cold-chain integrity and tight environmental ...
-
Product A vial machine for different formats
Schubert-Pharma has many years of experience and expertise in packaging vials, ampoules, syringes and other products for the pharmaceutical and healthcare industry. The rationale behind the development of the new machine was to enable manufacturers to meet line clearance requirements even more effectively....
-
Product Biopharmaceutical packaging
Blow-Fill-Seal (BFS) technology has been used to package liquid and semi-solid pharmaceutical products since the 1960’s. Our advanced aseptic process uses plastic polymer material to form, fill and hermetically seal a container in a highly integrated process. Various waters and small molecule drugs are ...
-
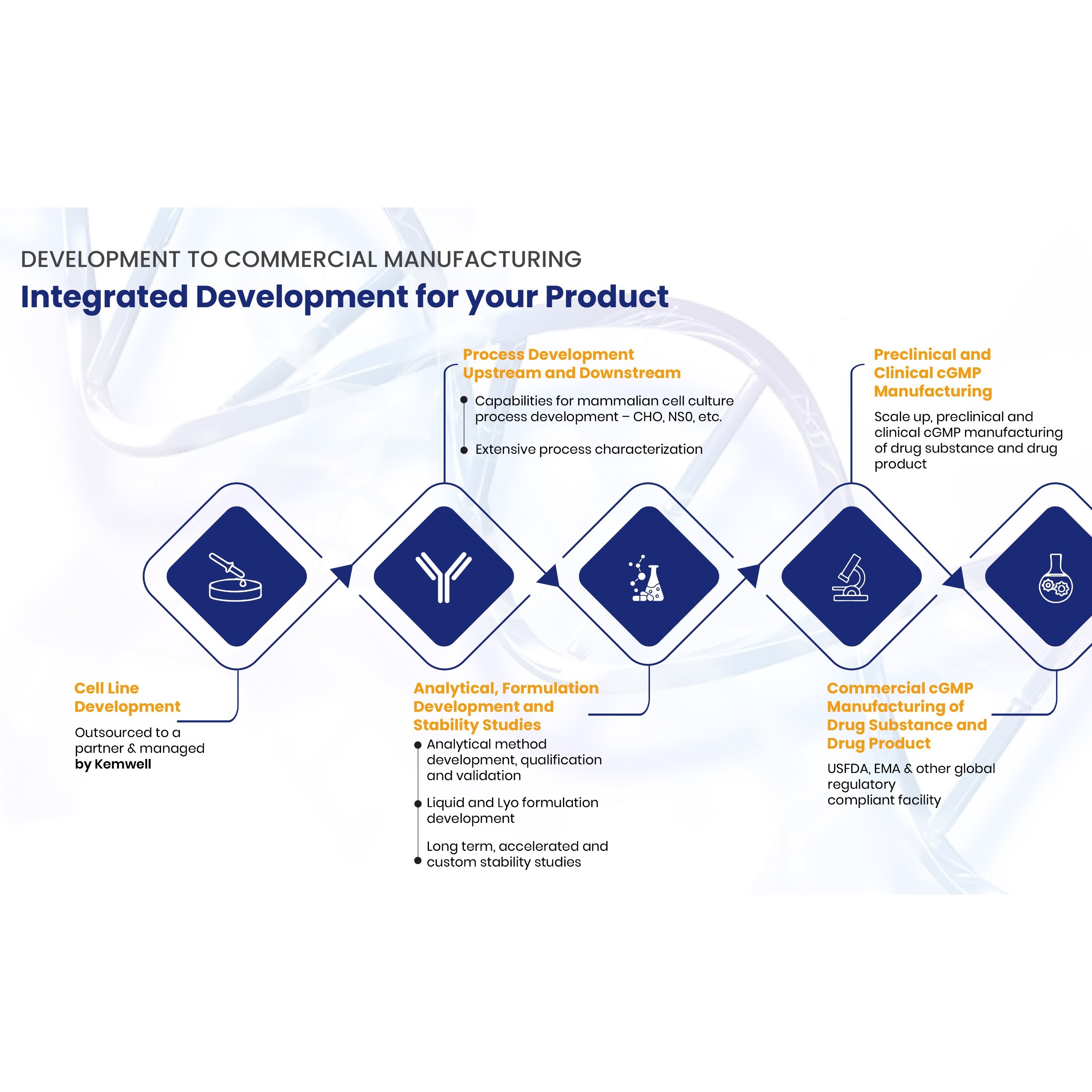
Product Kemwell Biopharma
SERVICES Kemwell provides integrated development and manufacturing services for companies that require one-stop solution for mammalian cell-culture based protein therapeutics The team is experienced to undertake end-to-end activities right from cell line development till cGMP clinical and commercial manufa...
-
Product Rituximab
Rituximab binds itself to the CD20 protein, marking them, and subsequently triggering the cells of the immune system to pick out the marked cells and kill them.The indication is for Non Hodgkin Lymphoma
-
Product Biologic drug product CMO services - Fill and Finish
GC Biopharma is one of the top-tier biopharmaceutical companies in South Korea with over 55 years of history and advanced technology. GC Biopharma provides premium Fill & Finish service to clinical and commercial customers for vaccines, recombinant and biosimilar pharmaceutical products. We have state-of-t...
-
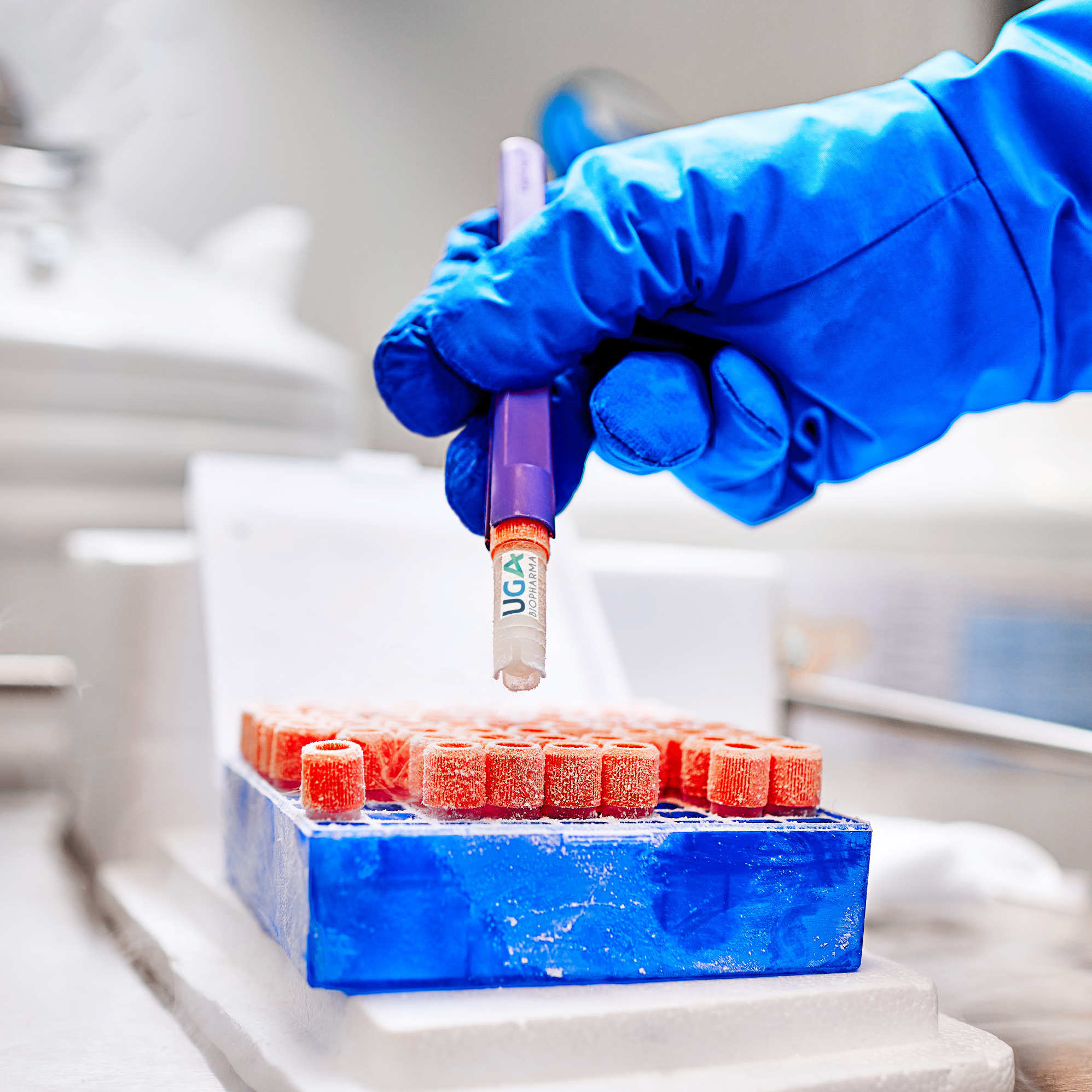
Product Ready-to Use CHO cell lines
UGA Biopharma GmbH offers to their clients so called Ready-to-use Biosimilar Cell Lines. This are in-house developed CHO cell lines stably expressing different biosimilar molecules, that are instantly available for in-licensing. The client profits, because the cell lines are directly available, and develop...
-
Product Darwin - Machine Performance Finder
Darwin, the Machine Performance Finder, offers various intelligent features to identify concrete optimization potentials of an automated machine. In this way, it significantly increases the performance level of the machines.
The Machine Benchmark learns a virtual ideal process on the basis ...
-
Product Variable Volume Micropipettes by CAPP
Ecopipette line of single-channel pipettes is designed to take manual liquid-handling workflows to a whole new level.Ecopipette autoclavable pipettes are crafted from the best materials, each single-channel pipette features a fully autoclavable body (except for the volume controller knob) to minimize the t...
-
Product Computer System Validation (CSV) Solutions
ProPharma's validation professionals leverage the latest risk-based Computer System Validation (CSV) and Computer Software Assurance (CSA) techniques to ensure that our clients' systems are ready for inspections from the FDA, EMA, MHRA, and other regulatory agencies. Our consultants have extensive experien...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





.jpg)