Market News
Market news
-
News Vibalogics set for live biological filling boost
The CDMO has expanded its capabilities with a new automatic vial filling line to meet increased demand. -
News New e-nose sniffs out rare progressive lung disease
The artificial nose can sniff out Pulmonary Arterial Hypertension on a person’s breath as the disease alters its signature. -
News SGS opens new dedicated facility for extractables and leachables testing in Wiesbaden, Germany
The new facility will be the global Center of Excellence for Extractable Studies and Impurities Profiling, as a hub across the global network of laboratories. -
News I Holland to hold webinar on how to overcome tabletting challenges
Company will discuss common tabletting problems such as increasing output without capital expenditure, eliminating sticking and abrasion, minimising headwear and corrosion, all of which can be done through innovative means. -
News Recipharm establishes long-term supply agreement with Tillotts Pharma
The agreement comprises technology transfer and commercial manufacturing of the products Entocort and Asacol. -
News PaxVax inks Swiss marketing and distribution agreement with Seqirus for influenza vaccines
With the addition of Agrippal and Fluad, PaxVax is building a robust portfolio of vaccines for the Swiss market. -
News Avantor Performance Materials and Nusil Technology to merge
Merger creates global leader in life science and advanced technology materials. -
News Stimwave’s drug-free wireless pain relief neurostimulator changing the lives of people suffering from chronic pain
World’s first wireless, miniature implantable device hailed by doctors and patients as a long-term therapy breakthrough. -
News Sponsors and CROs agree that integrating statistical modeling and knowledge of past performance into feasibility analyses improves accuracy of results
Informing the pharmaceutical drug development, manufacturing and commercialization industry. -
News Mylan to launch first generic to EpiPen auto-injector
List price of $300 per generic EpiPen two-pack carton, which represents a discount of more than 50% to the Mylan list price. -
News Shire's first prescription eye drop, Xiidra 5% now available in the US
Xiidra is the only prescription eye drop approved by the FDA for the treatment of both signs and symptoms of dry eye disease. -
News Elite announces development and license agreement with SunGen Pharma
Companies will collaborate to develop and commercialize four generic products - two CNS stimulants and two beta blockers. -
News RedHill Biopharma receives approval of a European patent supporting RHB-104 for MS
Patent expected to be valid until 2032, once granted. -
News Impax Laboratories issues voluntary, nationwide recall for one lot of Lamotrigine orally disintegrating tablet 200 mg
Potential for 100 mg blister cards being packaged in 200 mg containers. -
News NICE recommendation could save the NHS millions
Approximately 40,000 women in England seek hospital treatment for uterine fibroids every year. -
News Overcoming the challenges of implementing serialization across a diverse pharmaceutical manufacturing environment
New Webinar Hosted by Xtalks. -
News Mylan invalidates two of Teva's Copaxone 40 mg/mL patents
USPTO has ruled in favour of Mylan in its inter partes review proceeding and found all claims of two related Copaxone 40 mg/mL patents to be unpatentable. -
News Recipharm increases lyophilisation capacity in Italy
The investment will see the introduction of a new lyophiliser for vials, increasing the facility’s capacity by approximately 20%. -
News Mylan taking immediate action to further enhance access to EpiPen Auto-Injector
Mylan doubling the eligibility for patient assistance, effectively eliminating out-of-pocket expense for uninsured and under-insured patients. -
News Diabetes and obesity therapeutics market will more than double to $163.2 billion by 2022
A number of pipeline T2DM therapeutics are anticipated to be significant contributors to market growth over the forecast period, with some even expected to reach blockbuster status. -
News CPHI’s Pre-Connect Congress outlines current trends in pharma
Patient centricity, combination products, packaging innovation and biotherapeutics showing promise at the forefront of the industry’s development. -
News Mallinckrodt locates growing specialty brands businesses in Bedminster, NJ
Will invest more than $80 million. -
News Sagent Pharmaceuticals initiates nationwide voluntary recall of Oxacillin for Injection, USP, 10 g
Recall due to presence of iron oxide particulate matter. -
News BioTechnique launches second phase of manufacturing partnership
Under this partnership, BioTechnique will manufacture a commercially available vaccine product in a new, shelf-stable dosage form. -
News SciQuest gives customers full control over chemical inventory with latest ERM upgrade
Used by eight out of the top 10 global pharmaceutical companies, ERM’s newest version includes the industry’s first triple federated search engine. -
News Cytori Therapeutics receives Frost & Sullivan 2016 Technology Innovation Award
Recognized for innovation and advancements in cell therapy. -
News New guide explains how to achieve a healthy measurement system
Topics covered include why, how and when instruments should be calibrated to ensure they are delivering accurate measurement. -
News Major need for safer antiarrhythmic drugs will continue to plague atrial fibrillation market
Despite the restoration and maintenance of sinus rhythm being a common practice in the management of atrial fibrillation, the available antiarrhythmic drugs are of modest efficacy and have unfavorable safety profiles. -
News Regeneron announces agreement with BARDA for the manufacturing and testing of new antibodies against MERS virus
A component of Regeneron's Rapid Response program, which is also targeting Zika and Ebola. -
News Pfizer to acquire Medivation
Medivation agrees to transaction valued at $81.50 per Medivation share in cash, for a total enterprise value of approximately $14 billion. -
News Chronic pain treatment pipeline could yield innovative alternatives to opioids
GBI Research’s analyses identifies 129 first-in-class programs in active development. -
News Zenith Technologies expands US presence
Expansion is the result of the demand for the company's automation, process control and serialization technology services. -
News Indian FDA approves Innovus Pharma's Zestra, Zestra Glide, EjectDelay and Sensum+
Products now approved in seven countries. -
News Cerulean to slash its workforce
Cut in staff due to failed clinical study of CRLX101. -
News Evolva enters partnership on APIs
Partner to fully fund development of new production routes for a family of APIs. -
News Vedanta Biosciences granted US patent
Patent broadly covers pharmaceutical compositions for microbiome therapeutics based on bacterial consortia. -
News Hikma launches Levoleucovorin for Injection in the US
Product is therapeutically equivalent to Spectrus Pharmaceuticals' reference listed drug Fusilev Injection. -
News Turkey’s pharmaceutical industry set to hit $5.53 billion by 2020
The vast reforms seen throughout Turkey’s pharmaceutical market create a number of opportunities for pharmaceutical manufacturers. -
News Cystic fibrosis therapies will push Vertex ahead of GSK in $46.6 billion respiratory market by 2022
Transmembrane conductance regulator modulating therapies - of which there are currently only two and both are developed by Vertex - are set to have a substantial clinical and commercial impact. -
News Pharma and medtech sectors struggle in first half of 2016
Falling stock markets, political uncertainty and continued global economic weakness all contributed to a mixed first half, says market intelligence firm, Evaluate. -
News Hikma signs exclusive licensing, distribution and supply agreement with Basilea for Cresemba
Cresemba (isavuconazole) is an injectable and oral anti-fungal product. -
News Fresenius Kabi announces major investment in US manufacturing
State-of-the-art pharma manufacturing campus will be a showcase for the company and the production of injectable generics. -
News Unmet needs in rheumatoid arthritis treatment could be addressed by first-in-class pipeline
Cytokine and cytokine receptors, as well as protein kinases, largely dominate. -
News Piramal Enterprises to acquire CDMO Ash Stevens
Acquisition will provide Piramal with HPAPI capabilities in North America. -
News New report shows price increases for orphan medications correlated with non-orphan use
Trend could jeopardize the affordability of life-saving orphan drugs for patients with rare diseases and conditions. -
News Integra introduces new motorized tip spacing pipettes
The VOYAGER II is the only commercially available pipette range where the tip spacing can be changed electronically, -
News Bavarian Nordic and BMS announces drug supply agreement for NSCLC clinical study
Trial will look to explore the benefit of combining CV301 with Opdivo in patients with previously treated non-small cell lung cancer. -
News Irvine Scientific introduces BalanCD HEK293 system for large-scale expression of proteins and viral vectors
New product range includes chemically-defined, serum-free media, to increase productivity of viral vectors and recombinant proteins in suspension cultures. -
News Lonza extends its reach in the healthcare continuum with acquisition of InterHealth Nutraceuticals
Strengthens Lonza’s specialty nutritional ingredients portfolio. -
News SRF to invest in new chloromethane plant
New plant aims to double SRF’s capacity for chloromethanes to 80,000 tonnes per annum. -
News Arch Biopartners engages Catalent to begin manufacturing process for AB569
Preparations underway for the first human trials involving patients with antibiotic resistant lung infections. -
News Allergan to acquire eye care company ForSight VISION5
Acquisition adds global ights to non-invasive ocular ring in development for glaucoma and other eye conditions. -
News Novel lipid altering therapies will steer acute coronary syndrome market beyond $12 billion by 2022
Growth attributed to launches of the first proprotein convertase subtilisin/kexin type 9 (PCSK9 inhibitors), Repatha and Praluent by 2016, the launch of ETC-1002 by 2021, and increases in the global prevalence of ACS. -
News CPHI & P-MEC China explores emerging trends in biopharm and pharma packaging
New regulations speeding up growth with exports to Japan targeted. -
News CPHI Worldwide announces the board of Judges for the CPHI Pharma Awards 2016
CPHI Pharma Awards’ panel doubles in size and welcomes top experts from around the world. -
News Celsion and Zhejiang Hisun sign technology transfer, manufacturing and commercial supply agreement for the development of its GEN-1 immuno-oncology therapy
Expanded partnership provides capacity and cost structure supporting Celsion's global commercial strategy for GEN-1. -
News Agilent to build new facility in Colorado, increasing pharmaceutical manufacturing capacity
Expansion will enable the company to more than double its commercial manufacturing capacity for nucleic acid APIs. -
News Automation-ready blowdown evaporator available from Porvair Sciences
The Ultravap Levante will appeal to the budget-conscious laboratory who may wish to automate their sample dry-down bottleneck in the future. -
News New entrants will propel ophthalmological disorders market to $26 billion by 2022
Angiogenesis modulators and gene therapies to boost overall revenue within the forecast period. -
News Biogen announces Bioverativ as name of new hemophilia-focused company
Bioverativ will focus on accelerating innovation for people living with hemophilia and other blood disorders. -
News Microbiological environmental monitoring features in two national conferences supported by Cherwell Laboratories
Cherwell to offer expert insight in environmental monitoring and process validation to both NHS and industrial pharmaceutical manufacturers. -
News Vonvendi, the first and only recombinant treatment for adults affected by von Willebrand disease, launches in the US
Vonvendi is a significant advancement for the treatment of adults with von Willebrand disease. -
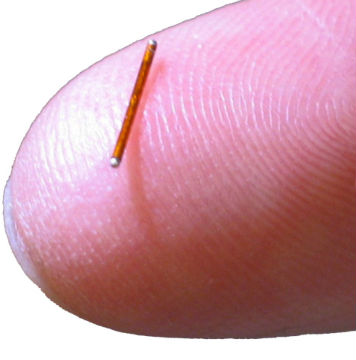
News University of Pittsburgh licenses novel microneedle patch to SkinJect
SkinJect Microneedle Patch to deliver drug to treat and immunize skin cancer patients. -
News Charles River Laboratories announces partnership with the Milner Therapeutics Institute and Consortium
Through this partnership, the academic institutions in the Consortium will have access to Charles River’s early discovery and drug development resources and services to accelerate their early-stage drug development processes. -
News Dr Reddy’s completes acquisition of product portfolio from TEVA
The portfolio is a mix of six ANDAs pending approval, one approved ANDA and one ANDA with tentative approval, and comprises complex generic products across diverse dosage forms. -
News SMC ‘U-turn’ offers lifeline to Scottish skin cancer patients
Opdivo (nivolumab) now recommended to treat Scottish NHS patients with advanced skin cancer. -
News Norgine and Valeant sign licensing agreement for NER1006 in US and Canada
Valeant obtains the rights to develop and commercialise NER1006 Powder for Oral Solution. -
News Mylan completes acquisition of Meda
With the addition of Meda, Mylan now has six $1 billion therapeutic franchises. -
News FDA approves first generic version of influenza drug
Natco receives approval for generic Tamiflu. -
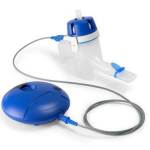
News The world’s first smart insulin pens with automatic wireless data transfer
Fully automated data transfer improves diabetes management. -
News FDA approves Flonase Sensimist Allergy Relief
Another Rx-to-OTC Switch from GSK Consumer Healthcare to help allergy sufferers find more complete relief. -
News AmpliPhi granted Japanese patent covering the use of phage therapy to resensitize Pseudomonas aeruginosa infections to antibiotics
AB-PA01 designed to broadly target both CF and non-CF Pseudomonas isolates and potentially eradicate the infection instead of merely keeping it at bay. -
News Vaxil announces key US Patent and Trademark Office Notice of Allowance for its immunotherapy
First US Patent issued to Vaxil since the company began developing its immunotherapies 10 years ago. -
News Astellas transfers US manufacturing subsidiary to Avara
Subsidiary will be renamed Avara Pharmaceutical Technologies. -
News Newer therapies will steer type 2 diabetes market in South-East Asia to $2.7 billion by 2022
Increase will be driven by the growing use of recently approved and emerging branded T2DM therapeutics, in favour of lower-cost generic products, says GBI Research. -
News Allergan announces sale of Anda distribution business to Teva Pharmaceuticals
Continues Allergan's evolution into focused brand growth pharma leader. -
News Regeneron and Adicet Bio collaborate to discover and develop next-generation engineered immune cell therapeutics
Companies to pursue "off-the-shelf" cellular therapies in oncology. -
News Allergan completes divestiture of global generics business to Teva
Sale advances company's evolution into a focused branded growth pharma leader. -
News Pfizer aims to become industry leader in gene therapy with acquisition of Bamboo Therapeutics
Acquisition combines Bamboo’s gene therapy portfolio, advanced vector design, and production technologies and capabilities with Pfizer’s global scale, research, development and commercialization experience. -
News Rare cancer drug which significantly delays progression of advanced thyroid cancer is left off relaunched Cancer Drugs Fund
People with radioiodine refractory differentiated thyroid cancer are overlooked as Lenvima is not scheduled for evaluation by NICE or the new Cancer Drugs Fund. -
News Venous thromboembolism therapeutics market set to hit $3.7 billion by 2025
Steady growth will be driven by the uptake of novel oral anticoagulants, which represent important advances over warfarin, a cheap and established anticoagulant, says GlobalData. -
News Merck to provide Provantage End-to-End development and manufacturing services to Y-mAbs for lead antibody compound
Includes scale-up and GMP manufacturing of drugs in late-stage development. -
News Tokai Pharmaceuticals announces reduction in force
Reduction is designed to reduce operating expenses while the company conducts a comprehensive evaluation of strategic options for galeterone and its pipeline. -
News Sunovion submits NDA for SUN-101/eFlow to FDA for the treatment of patients with COPD
If approved, SUN-101/eFlow will be the first nebulized long-acting muscarinic antagonist (LAMA) for the long-term, maintenance treatment of airflow obstruction in patients with COPD. -
News Slovakia-based CDMO sells registration dossiers for more than 20 pharma products
The portfolio of products will be marketed by Xantis Pharma throughout Central and Eastern Europe. -
News Sanofi receives FDA approval of Adlyxin for treatment of adults with type 2 diabetes
Adlyxin is approved as Lyxumia in more than 60 countries. -
News Codexis completes obligations under R&D agreement with leading biopharmaceutical company
Enzyme developed with CodeEvolver technology for preclinical therapeutics program exceeds agreement requirements. -
News Vectura initiates legal proceedings against GSK
GSK accused of patent infringement. -
News Cytokinetics and Astellas announce option right for tirasemtiv and expansion of global collaboration for CK-2127107 in ALS
Cytokinetics to receive $65 million in upfront payments and $30 million in additional sponsored R&D; potential for more than $100 million in payments associated with the exercise of the option. -
News NeuroDerm issued a new US patent for aqueous apomorphine covering concentrated subcutaneously delivered pharmaceutical compositions
Company's ND0701 formulation intended for chronic therapy of Parkinson's disease and is developed for continuous subcutaneous administration by a small, low-volume, disposable patch-pump. -
News GSK announces significant new investment in UK manufacturing network
Investment in advanced manufacturing of new respiratory and biopharmaceuticals portfolio. -
News John C. Lechleiter to retire as Lilly CEO; Board elects David A. Ricks as successor
Ricks will assume President and CEO roles on 1 January 2017, and become chairman of Lilly's board on 1 June 2017. -
News Top 3 therapy areas account for 68% of overall pharmaceutical industry pipeline
Oncology, infectious diseases and central nervous system disorders accounted for a combined 68% of the overall pharmaceutical industry pipeline as of Q1 2016, says GBI Research. -
News Ocular Therapeutix receives CRL from FDA for its NDA for Dextenza
FDA reports deficiencies in manufacturing process and controls identified during a pre-NDA approval inspection of the Ocular Therapeutix manufacturing facility. -
News Innova Biosciences introduces InnovaCoat GOLD-Carboxyl nanoparticles
Functionalized gold nanoparticles for easy, fast covalent conjugation of antibodies. -
News Shire launches pediatric indication for immunodeficiency treatment HyQvia in Europe
HyQvia is the only subcutaneous IG treatment for primary and certain secondary immunodeficiencies that can be administered in one site, once a month. -
News N-of-One Secures $7 Million in Series B financing
The financing will be used to expand the company's leading precision medicine decision support solutions in oncology. -
News RedHill Biopharma receives additional US patent covering RHB-105 ahead of confirmatory Phase III study for H. pylori infection
Company has received a Notice of Allowance for an additional U.S. patent covering RHB-105, expected to be valid until 2034 once granted. -
News BMS announces availability of FDA-approved Orencia ClickJect
New Orencia ClickJect Autoinjector offers accurate dose self-injection with push button operation, and confirmation that the full dose has been injected. -
News Despite 330 pipeline products, stem cell therapies face commercial challenges
The stem cells field is still surrounded by a wide variety of obstacles, most notably the high cost of research, according to key opinion leaders. -
News Recipharm appoints new General Manager in Research Triangle Park
Ann Flodin will be responsible for managing daily operations in Recipharm in Research Triangle Park and will focus on expanding and strengthening the development services Recipharm offers its customers in the US. -
News Lonza reports best first half in history with continued strong momentum
Pharma&Biotech’s outstanding operational performance across all assets bolstered the strong half-year results. -
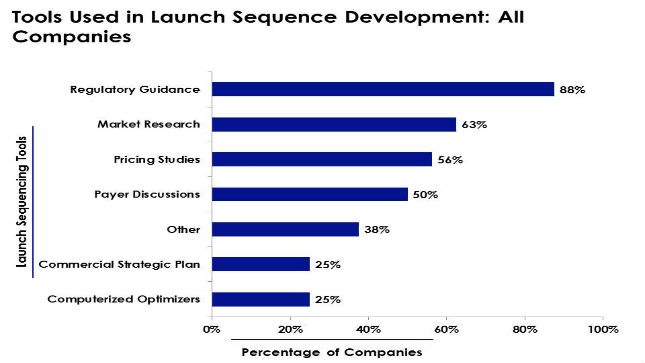
News Study finds 88% of pharma companies base global launch planning on regulatory affairs guidance
Various tools help drug companies establish successful global launch plans. -
News Responding to customer needs with ergonomic design
Enhancements to Thermo Scientific Finnpipette F1 and F1-ClipTip pipetting systems improve comfort. -
News Pfizer Receives WHO prequalification for multi-dose vial presentation of Prevenar 13
Designation will enable increased access to vaccine in world’s poorest countries. -
News Vietnamese pharmaceutical market to reach $6.6 billion by 2020
While the Vietnamese market is seen as attractive for pharmaceutical companies, drug prices are high compared with average incomes, says GlobalData. -
News FDA expands indication for type 2 diabetes treatment Synjardy
Expanded indication now includes treatment-naïve adults. -
News Dr Reddy's launches Omeprazole and sodium bicarbonate capsules in the US market
Product is a generic version of Zegerid - an acid reflux treatment. -
News Teewinot Life Sciences secures first US patent covering an apparatus for the biosynthetic production of authentic THCA, CBDA And CBCA cannabinoids
The apparatus facilitates the manufacture of authentic cannabinoids that are purer in form and more cost effectively produced than what is currently available in the marketplace. -
News Merck relaunches customer collaboration centers with new concept and M Lab name
Enables explorations of new technologies and solutions to address development and manufacturing challenges. -
News Advanced Proteome Therapeutics formulates strategic plan featuring novel antibody program
The company is focusing on enabling technology, designed to link antibodies to drugs or toxins to produce pure and homogeneous ADCs. -
News Vetter plans to invest $320 million on 10-year expansion plan
Company provides insights into its long-term expansion plans that include the purchasing of property in the City of Des Plaines -
News Sartorius acquires ViroCyt
Acquisition expands bioanalytical portfolio of Lab Products & Services division. -
News MRC Technology monetises royalties on cancer drug Keytruda to expand medical research activities
The charity will now also be able to pursue exciting novel research and development collaborations with new partners. -
News Amgen and Daiichi Sankyo announce agreement to commercialize biosimilars in Japan
The deal includes several biosimilars in late-stage development, including biosimilars of adalimumab, bevacizumab and trastuzumab. -
News Saudi Arabia’s pharmaceutical market set to hit $6 billion by 2020 as nation’s wealth increases
This impressive expansion can be attributed to a growing population, rising wealth levels, and demand for patented pharmaceutical products, says GlobalData. -
News Avacta and Glythera collaborate to develop novel, potentially highly potent, drug class
The collaboration will evaluate the use of Avacta’s Affimer technology in combination with Glythera’s PermaLink conjugation chemistry. -
News Apax Partners to acquire the operating subsidiaries of Invent Farma
Invent Farma develops, manufactures and markets generic drugs.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






























































































.jpg)










