Market News
Market news
-
News KD Pharma acquires Switzerland-based cannabinoid manufacturer PhytoXtract
The global CMO plans to have a complete, licensed cGMP manufacturing facility online by the end of 2021 -
News Catalent boosts plasmid DNA manufacturing at Rockville with addition of ABEC fermenters
The company's custom single run single-use fermenters supplied on fast-track schedule to support flexible manufacturing of plasmid DNA -
News Bavarian Nordic receives $28 million Ebola vaccine order from J&J
The smallpox vaccine specialist will manufacture and deliver bulk drug substance of MVA-BN Filo vaccine during the second half of this year -
News Moderna and Catalent expand COVID vaccine manufacturing agreement
The extended collaboration comes after reaching the initial milestone of producing 100 million doses -
News Sterling Pharma seals acquisition of bioconjugation specialist ADC
By integrating its HPAPI capabilities with those at ADC Biotechnologies' Deeside facility, small molecule CDMO will be able to offer the development and manufacturing of toxin linkers -
News NextPharma completes purchase of Lonza lipid oral dosage form manufacturing sites
Ploermel and Edinburgh facilities employ around 390 permanent staff and will allow UK CDMO to expand its technology offering -
News TCR2 bets on lead candidate with new cell therapy manufacturing facility
The US facility in Rockville is expected to accelerate the company’s commercial-scale manufacturing timelines of gavo-cel -
News Lonza to support Pionyr Immunotherapeutics' oncology drug development
The CDMO will provide cell line development, process development, drug substance and drug product manufacturing for Pionyr’s monoclonal antibody candidate -
News KD Pharma expands CDMO capabilities via asset acquisition
The former Rohner's manufacturing assets will enable omega-3 heavyweight to manufacture difficult, multi-step pharmaceutical intermediates and APIs -
News GSK to manufacture 60 million doses of Novavax vaccine for the UK
Pharma company will provide fill and finish manufacturing capacity at its Barnard Castle facility as early as the beginning of May 2021 -
News Nutrition additive supply chains 'nervous' as Suez blockage continues
Some seaborne deliveries are being rerouted or switched to air transportation as efforts to re-float stricken Ever Given container vessel move into fourth day -
News CURE Pharmaceutical gains DEA go-ahead to research psychedelics compounds
Company will use extension of Schedule I licence to start development of psychedelics-based pipeline to treat mental health disorders -
News China's Raybow Pharmaceuticals invests $15.8 million to expand US facility
The company will triple its capacity and workforce at its North Carolina site to increase its global capabilities and reach -
News Catalent signs ODT technology development agreement with Cybin
The CDMO's Zydis technology will help the Canadian biotech to develop fast-acting, shorter-duration formulations of its potential therapy for treatment-resistant psychiatric disorders -
News Wuxi Vaccines' Ireland site still on course for 2022 despite COVID challenges
Despite COVID-related challenges and supply chain issues, the CDMO's $240 million new facility is on target to open next year -
News PCI Pharma Services expands Berlin cold chain clinical supply storage capabilities
The move ensures the service provider has the capacity to meet the needs of an evolving clinical market -
News Growing drug delivery CDMO Kindeva expands UK operations
US-headquartered company Kindeva seals deal for an R&D facility at Loughborough’s life sciences hub -
News European countries suspend use of AstraZeneca COVID-19 vaccine as regulators investigate blood clot reports
Regulators stress temporary halt is precautionary measure and that there is currently no evidence linking the shot to adverse events -
News Growth opportunities abound for auto-injectors amid patient-centric healthcare transition, experts tell CPHI webinar
Tremendous growth opportunities exist for auto-injectors as their value is increasingly being recognised across several therapy areas, experts have told a recent CPHI webinar, sponsored by Stevanato Group. -
News ANI Pharmaceuticals expands CDMO business through Novitium acquisition
The purchase will allow the company to add nine new customers to its growing CDMO business and 27 manufacturing suites -
News Inhalation specialist Vectura invests to expand manufacturing capabilities
The enhancements will include installing the lever-operated multi-dose inhaler device used in Hikma’s generic Advair product, which the FDA recently approved -
News CPHI post-pandemic report forecasts explosion of contract services growth
During the next 12 months, demand for early-stage contract projects could quickly exceed supply -
News Lonza to develop and manufacture Ixogen's oncolytic virus
PsiVac has generated compelling efficacy data in killing a broad range of tumour cells, including head and neck, bladder, liver, pancreatic and ovarian -
News Merck agrees to support Johnson & Johnson in vaccine manufacturing deal
Funding from BARDA helps the company to adapt and make available a number of existing manufacturing facilities for the production of SARS-CoV-2 vaccines and medicines -
News WuXi AppTec completes $135 million acquisition of Oxgene
The addition further strengthens WuXi's cell and gene therapy service offerings for global customers and will be Wuxi ATU's first facility in Europe -
News Evonik scoops CMO Leadership Award for fourth year in a row
The company wins awards in all six categories — capabilities, compatibility, expertise, quality, reliability and service -
News Pharmaron pays $118.7 million for AbbVie UK biomanufacturing site
This transaction complements Pharmaron’s recent acquisition of Absorption Systems in the US for building an integrated cell and gene therapy services platform -
News Catalent takes double action to boost cell and gene therapy services
The company acquires a pDNA CDMO and launches pDNA development and manufacturing services at its Rockville, Maryland facility -
News BMS expands global footprint with new cell therapy manufacturing facility
The new site will be equipped to enable quick ramp-up of capacity for clinical and commercial cell therapy products -
News Catalent secures softgels commercial supply agreement with Aurinia
The CDMO will manufacture recently FDA-approved Lupkynis as a softgel dosage form at its St Petersburg, Florida, facility -
News MaxiVAX and Minaris strike immunotherapy manufacturing partnership deal
The manufacturing agreement includes process development for scale-up and technology transfer with the goal of supplying Phase II and III clinical studies -
News Wavelength takes majority stake in Vanamali to strengthen drug substance manufacturing
API manufacturer says acquisition is next step of investment strategy to closely align API supply to customer needs in an unpredictable world -
News Cascade Chemistry starts $14 million API manufacturing expansion
CDMO says increased customer demand for clinical API supply has outstripped its cGMP manufacturing capacity -
News Regis completes expansion of US-based API development facility
The CDMO doubles its capacity to take on new development projects -
News Lonza boosts solid form services for small molecule APIs
The expanded and refined first-in-human services have been designed to meet the early-stage molecule development of small biopharma players -
News Phlow secures $20 million to advance manufacturing initiatives in the US
The company seeks to actively accelerate US manufacturing for medicines at risk of shortage, including those for COVID-19 pandemic response -
News Dermapharm expands COVID-19 vaccine production capacity at ex-Merck site
The company's additional production capacities are scheduled to go into operation in May 2021 -
News AstraZeneca partners with IDT Biologika to build COVID-19 vaccine facility in Germany
Firms plan to invest in capacity expansion at the CDMO’s manufacturing site in Dessau to boost European domestic vaccine supply -
News Clinical knowledge deficits to slow biotech development, warns Europital
The CRO predicts investment communities will come to the rescue of small and medium companies looking to advance their candidates -
News Argonaut Manufacturing Services opens custom controls & standards facility
The CMO has designed the new service to alleviate manufacturing challenges associated with positive controls and nucleic acid templates -
News WuXi STA to grab foothold in Europe with planned BMS facility purchase
The acquisition will expand the CDMO's capabilities and capacity to serve European markets -
News Innovation in Drug Delivery - Part One: The trend towards self-administration
By Yifei Chen and Yasemin Bettina Karanis, IQVIA
-
News Innovation in Drug Delivery - Part Two: Therapy area trends for injectables
By Yifei Chen and Yasemin Bettina Karanis, IQVIA -
News Sepha launches small batch contract packaging service amid rising demand
The CPO's new division will offer a holistic blister packaging development service, complete with QA and non-destructive leak testing, encompassing material selection, tooling design, and 3D prototyping -
News COVID-19, cancer and competition: Key demand dynamics for the API sector
How are changes in new treatments and disease areas – as well as the current pandemic crisis - affecting demand for APIs? -
News EU delivers export threat after AstraZeneca cuts Q1 vaccine supply
European Commission pressures pharma firm over reduced shipments amid criticism for slow vaccine rollout across block -
News Emergent to manufacture Humanigen COVID-19 therapeutic
The CDMO gears up for rapid deployment of its clinical-to-commercial manufacturing operations for its seventh collaboration to help deliver COVID-19 treatments -
News Oxford University to create institute to fight antimicrobial resistance after Ineos donation
The donation from Ineos will create a new institute to tackle the "silent pandemic" -
News Sartorius and RoosterBio partner to advance scale-up of hMSC manufacturing
The collaboration aims to accelerate the development and commercialization of groundbreaking cell-based regenerative cures -
News Thermo Fisher buys Novasep viral vector manufacturing business
Expanded global capacity addresses growing demand for cell and gene therapy -
News Merck KGaA expands its mRNA capabilities through AmpTec acquisition
AmpTec's differentiated PCR-based technology has shown to have advantages compared with other technologies for mRNA manufacturing -
News Abzena to open new biologics GMP manufacturing site in the US
The new facility will house up to 12 X 2000-L bioreactors and will be equipped to handle existing and new advances in manufacturing -
News Fujifilm Diosynth Biotechnologies to establish new viral vector manufacturing facility in Boston
The $40 million investment marks the company's third viral vector manufacturing site -
News Wuxi agrees €150 million deal to buy Wuppertal drug substance facility from Bayer
Acquisition will extend the Chinese CDMO’s European footprint and will complement Leverkusen drug product site -
News Lonza to expand bioconjugation capabilities at Visp site
CDMO will add two production suites for clinical and commercial supply and extend lab space to double analytical and process development capacity -
News Viatris to slash up to 9,000 jobs at manufacturing facilities as part of global cost-cutting plan
Newly formed generics business identifies the first five impacted plants as it seeks to reduce costs by $1 billion by the end of 2024 -
News Thermo Fisher boosts clinical supply chain and distribution services in Europe
Two new facilities in Germany will provide clinical supply and cold chain services to support growing demand -
News Lilly nabs gene therapy company Prevail Therapeutics
Through the acquisition, Lilly will establish a gene therapy program via Prevail's gain clinical-stage and preclinical neuroscience assets -
News Vectura expands capabilities to develop highly potent inhaled drugs
The expansion will enable the CDMO to widen the nature of inhaled drug development projects it can undertake for customers -
News AstraZeneca taps Halix for commercial drug substance manufacture of COVID-19 vaccine
Dutch CDMO will expand with two additional viral vector production lines to meet the increased demand -
News Catalent starts manufacture of Passage Bio's lead gene therapy products
The CDMO will provide packaging, labeling and distribution services through its FastChain demand-led supply offering -
News FUJIFILM Irvine Scientific introduces Sterile Express Media Service
The first-of-its-kind manufacturing service delivers tested sterile, non-GMP media designed for feasibility testing before scale-up, to support development of cell and gene therapies -
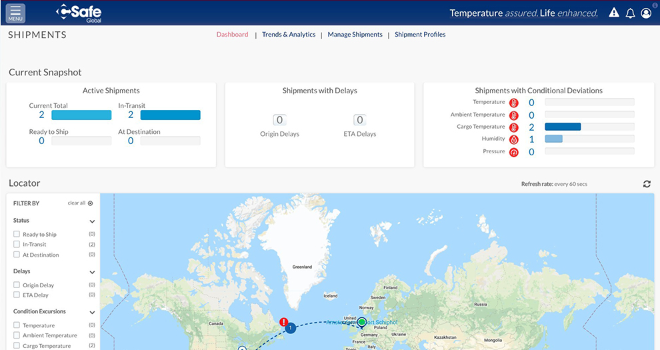
News CSafe Global launches real-time shipment visibility platform
Industry customers now have access to 24/7 real-time shipment visibility across the company's entire product portfolio -
News Lonza expands development and manufacturing capabilities at Bend site
The company will invest in dedicated early-phase development and cGMP manufacturing to help customers rapidly advance challenging molecules -
News AZ and Oxford COVID-19 vaccine brings end of pandemic a little closer
AZD1222 is 90% effective, cheap to produce and easy to store, distribute and administer -
News Recipharm signs up for aseptic fill-finish manufacturing of Moderna COVID-19 vaccine
The CDMO is already making certain investments to enable technology transfer and scale-up to commence imminently. -
News Alvotech and Yangtze River forge biosimilar deal for Chinese market
The partnership will focus on commercializing eight biosimilar medicines in China for the domestic market -
News Catalent signs commercial supply agreement with Blueprint Medicines
The agreement comes hot on the heels of FDA approval of Gavreto — Blueprint Medicines' new treatment for lung cancer -
News Early trial results hail Moderna's COVID-19 vaccine as 94.5% effective
Data also show that mRNA-1273 remains stable at 2–8°, the temperature of a standard home or medical refrigerator, for 30 days. -
News Seqirus to build $800m influenza vaccine manufacturing facility in Australia
The facility will be the only cell-based influenza vaccine manufacturing facility in the Southern Hemisphere. -
News European Commission wraps up CureVac COVID-19 vaccine supply deal
Europeans set to benefit from the EC's authorization of COVID-19 candidate vaccine supply agreement, securing up to 405 million doses of CVnCoV -
News Vibalogics establishes US presence with virotherapy facility in Boston
The specialist CDMO's $150 million 3-year investment plan will provide a complete suite of virotherapy services to support drug developers and their most "revolutionary" products. -
News Stelis starts next phase of investment into its biologics manufacturing facility
This phase sees the CDMO start work on installing two 2,000-L single-use bioreactor trains in its mammalian cGMP facility. -
News Pfizer and BioNTech COVID vaccine proves 90% effective
Results from a Phase III clinical study found Pfizer and BioNTech's mRNA-based vaccine candidate, BNT162b2, is more than 90% effective in preventing COVID-19 in participants without evidence of prior SARS-CoV-2 infection, the companies announced Monday... -
News Altimmune signs up Lonza to manufacture intranasal COVID-19 vaccine
Altimmune adds Lonza as a manufacturing partner for supply of AdCOVID, its single-dose intranasal vaccine candidate for COVID-19. -
News CDMOs benefit from COVID-19 impact, says CPHI Annual Report
CMO growth, however, hampered by innovator approvals from mega-cap pharma. -
News The challenges of innovating in packaging and drug delivery
In this interview with Pharmapack, Arnaud Steiner, R&D Packaging Manager at Virbac shares his thoughts on trending issues in the packaging market such as patient centricity and eco-design, the challenges of managing the development of new packaging and... -
News Nostrum Laboratories recalls lots of metformin tablets after nitrosamine detection
Company is the latest in a lengthening list of pharma companies recalling the diabetes medicine over unacceptable levels of NDMA -
News Pill Connect secures £0.5m to take electronic smart pill bottle to commercial launch
Device provides clinicians and trialists with comprehensive data on a patient's adherence pattern. -
News Minaris Regenerative Medicine to expand its cell & gene therapy manufacturing capacity
The expansion will entail building two new facilities - one in Germany, the other in Japan. -
News Pfizer to invest €300 million in Irish operations
Initial stage of new facility planned for Ringaskiddy site to manufacture medicines for clinical trials. -
News Sanofi and GSK to support COVAX with 200 million doses of COVID-19 vaccine
The companies anticipate first results of Phase I/II in early December 2020, to support the initiation of a pivotal Phase III study before the end of the year. -
News Pharmaceutical shortages open up debate on reshoring of API manufacturing
Panel at CPHI Festival of Pharma discusses options for companies to ensure robust supply chains and reduce overseas supply dependency -
News Russia submits Sputnik V emergency use and prequalification application to WHO
The Russian Federation has become one of the first countries to apply to the WHO for prequalification of its vaccine, Sputnik V, against the novel coronavirus infection. -
News Suspected cyber attack hits Dr. Reddys
Company closes "key" plants in India, the US, Russia and Brazil as a precautionary measure. -
News Sai Life Sciences to boost biology capabilities at its integrated R&D campus
The CRDMO will expand in vitro and in vivo biology services, DMPK and toxicology capabilities. -
News Lonza expands its capsule manufacturing capacity
The expansion at eight global Lonza sites will increase manufacturing capacity by 30 billion capsules annually. -
News RedHill Biopharma secures expanded manufacturing for COVID-19 therapeutic candidate
The biopharma company collaborates with European and Canadian suppliers for large-scale ramp-up of opaganib manufacturing. -
News Milan to host CPHI Worldwide return in 2021
2021 will bring global pharma community back together, in person, to drive business growth. -
News LLS Health signs partnership to develop one-year contraceptive vaginal system
The CDMO's Pathway TPUs are well-recognized as an effective option for many drug delivery systems. -
News Synthesis Theta
The recipe for efficiency by Universal Pack -
News Company Profile
MPA Technical Devices Srl is an Italian company specializing in designing and manufacturing top quality and accurate dosing systems. -
News Company introduction – Cyclolab Cyclodextrin Research and Development Ltd
CycloLab Cyclodextrin Research and Development Ltd. is a private SME with the focus on cyclodextrin research and development for over 30 years. We are working in the fields of pharmaceutical, cosmetic and food industry, agrochemical, environmental and ... -
News ATMPS's blockchain platform the world's first solution for personalized medicines?
New ‘blockchain ecosystem’ has potential to track, from vein-to-vein, advanced therapies. -
News Sirio Europe invests in advanced manufacturing and green technologies
Manufacturing facility upgrades have substantially increased the CDMO’s production capacity and energy efficiency. -
News Catalent launches OptiGelR DR, a delayed/enteric release softgel
The technology eliminates the processing and performance challenges associated with conventionally coated delayed-release softgels. -
News Recipharm to boost sterile liquids manufacturing capacity
The investment will support increased demand and help to secure new business at its Kaysersberg facility. -
News ProductLife Group acquires pharmacovigilance services provider
The strategic acquisition of Axpharma is part of the group’s aim to widen its expertise and global reach as the pandemic forces renewed focus on safety, quality and speed-to-market. -
News 17th Annual CPHI Pharma Awards winners announced
Excellence in Pharma recognised at virtual ceremony taking place at the CPHI Festival of Pharma. -
News Sharp invests $10M in biologics and injectables packaging equipment
Investment includes the installation two of automated top-loading cartoner with the flexibility to pack vials, syringes, pens/autoinjectors and multi-component kits. -
News Cambrex completes major expansion of solid form screening facility in Edinburgh
The expansion increases the company's capacity and provides its clients with larger process crystallization development, up to 20-L scale. -
News Biologics manufacturing and diagnostics need resiliency, says Scott Gottlieb
Former FDA Commissioner lays out roadmap for building out excess capacity to deal with pandemic situations -
News Abzena invests $60m into cGMP manufacturing capacity
The facility will enable the company to deliver Phase I to commercial manufacturing services for biologic projects. -
News CPHI Festival of Pharma Podcast - Day 4
The CPHI Festival of Pharma is well underway! Tune into this podcast in which Informa Markets Head of Pharma Content, Tara Dougal, and Pharma Editor, Gareth Carpenter review some of the highlights of the first few days as well as a preview of what's co... -
News MHRA approves second of two GMP manufacturing suites for COVID-19 vaccine candidate
The two suites, established with VMIC equipment and operated by Oxford Biomedica, will be operating at 1000-L scale. -
News HPNE introduces defined tubing routing for single-use facilities
Flexible support system simplifies and secures tubing paths for biopharmaceutical manufacturers. -
News Purification specialist BIA Separations to become part of Sartorius
BIA Separations will be Sartorius's center of excellence for purification of cell and gene therapeutics. -
News Cobra Biologics to manufacture plasmids for COVID-19 vaccine trial
Scancell’s DNA vaccine could provide long-lasting immunity against COVID-19 by generating protection against this and new strains of coronavirus. -
News Successful MHRA regulatory inspection for Scottish CMO Symbiosis
Inspection conducted remotely using video-conferencing and an online private document sharing platform. -
News Novel customizable and flexible single-use assembly solution
Hugh Purity New England's solution can utilize other vendors' components and can be implemented directly into any process. -
News New thin film technology to revolutionize vaccine storage and distribution
Up to 500 doses of vaccine to be placed on a single wafer-thin and stored for extended periods of time. -
News Sterling Pharma Solutions launches £11 million Material Science Centre
The new centre will allow the company to improve efficiencies and reduce overall manufacturing time. -
News New preventative nasal spray treatment could protect from COVID-19 infection
Novel therapy developed by Australian biotech company, Ena Respiratory, shown to significantly reduce COVID-19 virus levels in the nose and throat. -
News CPHI Pharma Index highlights the importance of international partnering
Survey results reveal 84% of the industry is actively searching for new partners. -
News Alvotech and DKSH extend biosimilar partnership in Asia
The companies will commercialize six new biosimilar candidates addressing multiple therapeutic areas. -
News Europital launches as a science-driven full-service CRO
Expanded CRO services to support biotechs and mid-sized pharma companies across trials in more than 40 countries. -
News Micreos initiates trial to evaluate world's first endolysin-drug as a therapy for atopic dermatitis
The targeted removal of one particular bacterial species from the skin microbiome, while preserving the beneficial ones, represents a new way to treat atopic dermatitis. -
News T-knife and Catalent sign tech transfer and manufacturing agreement
Catalent to manufacture clinical batches of T-knife's platform process for TCR-based cell therapy at its site in Gosselies, Belgium, for European trials in 2021.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




















































.png)






.jpg)