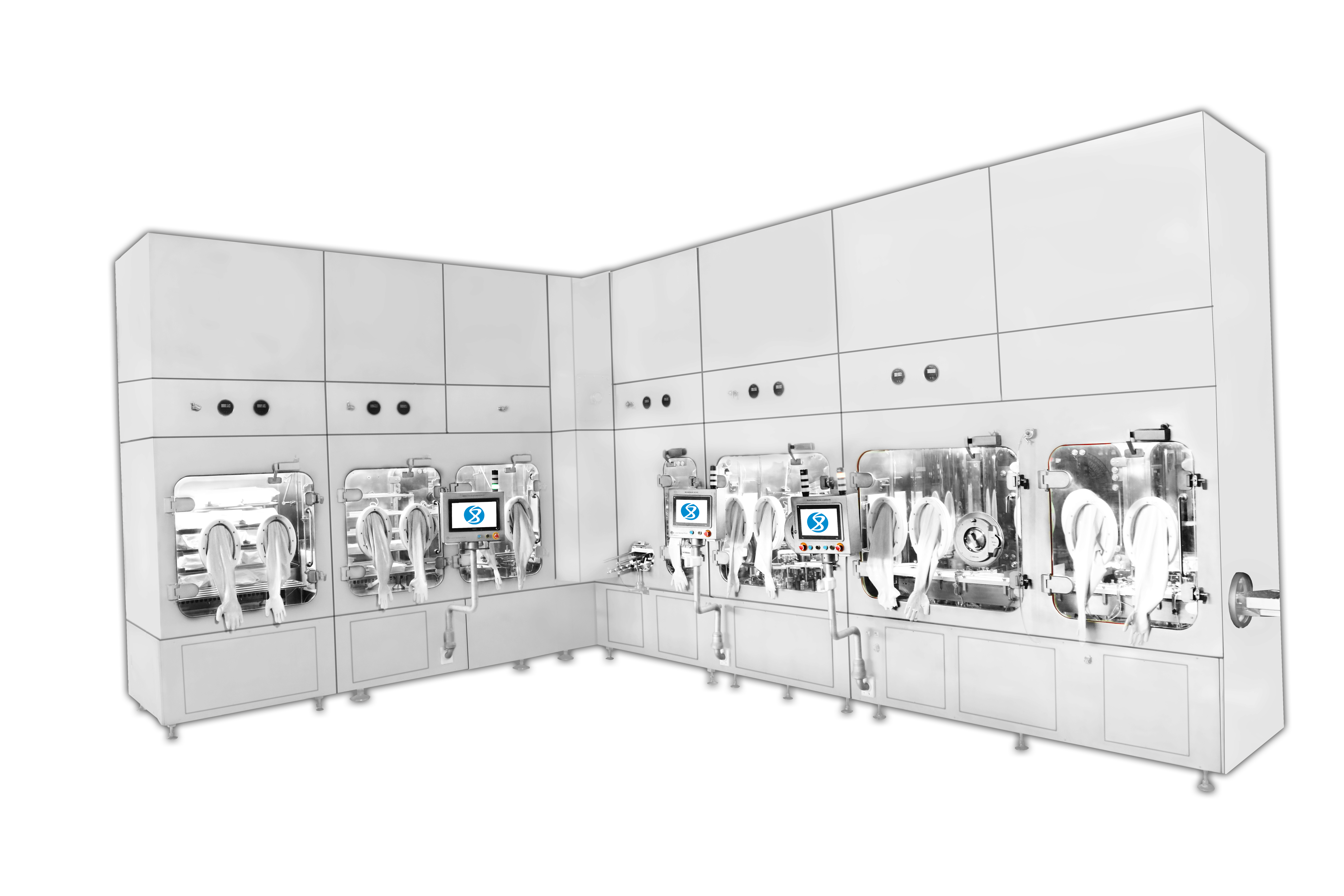
HPAPI Sterile Dispensing Isolator

Product Description
FPS Pharma

-
IT
-
2015On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
Primary activities
Categories
Specifications
FPS Pharma

-
IT
-
2015On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
Primary activities
More Products from FPS Pharma (41)
-
Product Isolator for Milling and micronization - Production Size
Milling operations may result in dust clouds, exposing operators and environment to HPAPI, due to high energy involved in process operations and size reduction down to few m.Proven solutions are available for laboratory, pilot unit or production units of: • Conical mills • Hammer mill • ... -
Product Sterile Contained PM8 Micronizing systems
The equipment, designed to be installed across different floors, is composed of a glovebox to discharge the batches from a filter dryer without affecting the product sterility; a jet mill isolator provides a confined environment in which the product is micronized; downflow booths are integrated on the ... -
Product Pack-off unit in Isolator
Pack-off systems assure product protection and reduce operator dust exposure during discharging operations from silos, bins, cyclones, driers, reactors, centrifuges, …
It is possible to install pack-off systems on existing equipment, reducing to a minimum the requested modification, in full compl... -
Product Multimilling Station in Isolator
Milling operations may result in dust clouds, exposing operators and environment to HPAPI, due to high energy involved in process operations and size reduction down to few μm.
Proven solutions are available for laboratory, pilot unit or production units of:
• Conical mills • Hamm... -
Product Multimilling Station
The multimilling station is the best choice for the execution of milling operations in research departments and pilot plants, with different technologies integrated on a single system.The platform considers:- • Spiral jet mill (type PILOTMILL-4, PILOTMILL-5 or PILOTMILL-6) • Pin mill 100 ... -
Product Sterile/Toxic Isolator
Containment systems for sterile applications may be proposed in the form of closed isolators or Restricted Access Barrier Systems (RABS).
A sterile isolator allows to avoid the use of sterile cleanrooms, reducing the sterile volume around the process being executed.
Turbulent or unidirectional fl... -
Product Spiral Jet Mills series - R&D and laboratory - PilotMill-Zero
PilotMill-Zero represents the right solution for preliminary feasibility micronization trials with milligrams batches of New Chemical Entities, as well as of many other products.
• Based on the proven FPS Spiral Jet Mill technology • Designed to effectively operate despite the miniaturized dimensions ... -
Product Spiral Jet Mills series - R&D and laboratory - LaboMill/PilotMill-1
The LaboMill/PilotMill-1 is the FPS answer to R&D lab needs when it comes to very initial stages of development, when only extremely small "batch sizes" are available. This very limited amount of material - starting from 200 mg - asks for the use of very special equipment designed and develop... -
Product Qmills series - R&D and laboratory
The R&D QMill isengineered to meet the requirements of R&D labs, where new product are developed and micronization process is requested on small quantities (from minimum 1g to few kgs).
All connections of components are made to be in compliance with cGMPs and to assure quick a... -
Product Spiral Jet Mills series - Pilot Unit - PilotMill-2
The PilotMill-2 jet mill is designed for product research and developments needs, where batches from 1g to 3kg are considered. In function of these process condition, the PilotMill-2 jet mill has been designed to assure higher micronized product yield. It is available in top discharge ... -
Product Spiral Jet Mills series - Pilot Unit - PilotMill-3
The PilotMill-3 jet mill still more indicated for R&D activities, can start micronizing batch sizes as low as 3g with extremely contained product loss and can micronize batches up to approx. 10kg. It is available in top and bottom discharge configurations, to better fit different needs.
... -
Product Spiral Jet Mills series - Pilot Unit - PilotMill-4
The PilotMill-4 jet mill is designed for product developments needs, where batches from 5g to 60kg are considered. It is available in top and bottom discharge configurations, to better fit different needs.
The PilotMill-4 jet mill may be easily integrated into glove box systems to ...
FPS Pharma resources (4)
-
Brochure Pharmaceutical Micronization and Milling Equipment
Explore FPS’ micronization and custom jet milling systems. Achieve exact PSD targets, enhanced bioavailability, and cGMP compliance for superior formulations. -
Brochure Pharmaceutical Isolators for Asepting Processing
Discover FPS’ isolators for aseptic processing. Ensure sterility, safety, and cGMP compliance, tailored to your production requirements and plant space. -
Brochure Pharmaceutical Containment Isolators for HPAPI Compounding and Processing
Discover how to optimize pharmaceutical HPAPI processing with FPS’ advanced containment isolators. Ensure top-tier safety, compliance, and efficiency. -
Brochure GloveXpert: The Innovative Glove Leak Tester for Quick, Easy & Reliable Testing
Discover GloveXpert: the ultimate glove leak tester for pharmaceutical isolators. Fast, accurate, ergonomic, and lightweight. Visit our booth to test it!
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




















.jpg)









-comp246544.jpg)

-comp285903.png)








































































%20(1)%20(1)-comp246058.png)