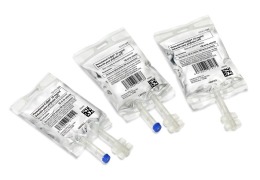
DP-Anastrozole (30 tablets/blister pack)

Product Description
Douglas Pharmaceuticals Ltd.

-
NZ
-
2015On CPHI since
-
500 - 999Employees
Company types
Specifications
Douglas Pharmaceuticals Ltd.

-
NZ
-
2015On CPHI since
-
500 - 999Employees
Company types
More Products from Douglas Pharmaceuticals Ltd. (99)
-
Product Clopine (25 mg/50 tablets/bottle packs)
CLOPINE (clozapine) is an atypical antipsychotic agent. Clozapine is chemically identified as 8-chloro-11-(4-methylpiperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine. Clozapine is a yellow crystalline powder. The PhEur monograph for clozapine solubility states that clozapine is freely soluble in methylene chlor... -
Product Clopine (50 mg/100 tablets/blister packs)
CLOPINE (clozapine) is an atypical antipsychotic agent. Clozapine is chemically identified as 8-chloro-11-(4-methylpiperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine. Clozapine is a yellow crystalline powder. The PhEur monograph for clozapine solubility states that clozapine is freely soluble in methylene chlor... -
Product Clopine (50 mg/100 tablets/bottle packs)
CLOPINE (clozapine) is an atypical antipsychotic agent. Clozapine is chemically identified as 8-chloro-11-(4-methylpiperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine. Clozapine is a yellow crystalline powder. The PhEur monograph for clozapine solubility states that clozapine is freely soluble in methylene chlor... -
Product Clopine (50 mg/50 tablets/blister packs)
CLOPINE (clozapine) is an atypical antipsychotic agent. Clozapine is chemically identified as 8-chloro-11-(4-methylpiperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine. Clozapine is a yellow crystalline powder. The PhEur monograph for clozapine solubility states that clozapine is freely soluble in methylene chlor... -
Product Clopine (50 mg/50 tablets/bottle packs)
CLOPINE (clozapine) is an atypical antipsychotic agent. Clozapine is chemically identified as 8-chloro-11-(4-methylpiperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine. Clozapine is a yellow crystalline powder. The PhEur monograph for clozapine solubility states that clozapine is freely soluble in methylene chlor... -
Product Clozapine 50 mg/ml Oral suspension
CLOPINE (clozapine) is an atypical antipsychotic agent. Clozapine is chemically identified as 8-chloro-11-(4-methylpiperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine. Clozapine is a yellow crystalline powder. The PhEur monograph for clozapine solubility states that clozapine is freely soluble in methylene chlor... -
Product DP-Anastrozole (30 tablets/bottle pack)
Anastrozole is a benzyltriazole derivative. The chemical name is a,a,a`a`-tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-benzenediacetonitrile. DP-ANASTROZOLE is used to treat breast cancer in women after they have undergone menopause. DP-ANASTROZOLE slows or stops the growth of cancer cells, but it does ... -
Product DP-Anastrozole (90 tablets/bottle pack)
Anastrozole is a benzyltriazole derivative. The chemical name is a,a,a`a`-tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-benzenediacetonitrile. DP-ANASTROZOLE is used to treat breast cancer in women after they have undergone menopause. DP-ANASTROZOLE slows or stops the growth of cancer cells, but it does ... -
Product DP-Lotion HC 1% (250mL)
DP Lotion-HC 1% is used as an aid to relieve the symptoms of inflammation such as redness, swelling and itching due to skin disorders. Hydrocortisone belongs to a group of medicines called corticosteroids. Hydrocortisone is 11ß,17a,21-trihydroxy-pregn-4-ene-3,20-dione. Indications: DP LOTION-HC 1% is indic... -
Product Egozite Cradle Cap Lotion 50ml
Egozite Cradle Cap Lotion has been formulated solely for the treatment of cradle cap. It It contains salicylic acid for a rapid and more complete treatment than the more traditional cradle cap remedies (of just oil). The keratolytic lotion helps loosen and remove the crusts of cradle cap and usually starts... -
Product ESTELLE-35 (28 tablets)
The chemical name of ethinyloestradiol is 19-Nor-17a-pregna-1,3,5(10)-trien-20-yne-3,17-diol, the chemical formula is C20H24O2 and the molecular weight is 296.4. Pack quantities: Three calendar packs of 21 tablets. Indications: ESTELLE-35 and ESTELLE-35 ED are indicated for the treatment of androgen depend... -
Product ESTELLE-35 (84 tablets)
The chemical name of ethinyloestradiol is 19-Nor-17a-pregna-1,3,5(10)-trien-20-yne-3,17-diol, the chemical formula is C20H24O2 and the molecular weight is 296.4. Pack quantities: Three calendar packs of 21 tablets. Indications: ESTELLE-35 and ESTELLE-35 ED are indicated for the treatment of androgen depend...
Douglas Pharmaceuticals Ltd. resources (1)
-
Brochure Douglas Pharmaceuticals - CDMO Partnering
An introduction to the partnering opportunities for softgel CMDO with Douglas.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance