Process Validation (Biopharmaceutical Services)
Process Validation (Biopharmaceutical Services) Companies (24)
Process Validation (Biopharmaceutical Services) News
-
News Thermo invests $50 million to enhance biologics capabilities
With the capacity expansion, Thermo Fisher will have the second largest base of single-use bioproduction capacity at a CDMO in the world.
Process Validation (Biopharmaceutical Services) Products (30)
-
Product GxP Audits and Inspection Readiness
Our auditors have real world experience supporting regulatory inspections and preparing companies for pre-approval inspections. Our team of specialists includes former regulatory agency inspectors and qualified auditors who are proficient in conducting mock inspections, internal audits, vendor and s...
-
Product GO!FIVE Digital Validation Solution
Global Verification & Validation is a SaaS solution for Life Science companies that complies with EMA/FDA. FIVE Validation developed a SaaS platform that allows AI and traditional validation studies to proceed 6x faster than a manual (electronic or paper-based) process.
-
Product Validation
Our team of experts will prepare necessary protocols incorporating all critical parameters
Process Validation:
PharmEng professionals possess experience in validating pharmaceutical and biopharmaceutical manufacturing processes. Our team of experts will prepare necessary protocols i...
-
Product Oral Solid Doses
Synerlab is a well known and highly regarded producer of dry forms specializing in small, medium and large production batches runs. We offer a wide range of technologies, including tablets, coated and sugar coated tablets, hard and soft gelatin capsules, in different packaging options (blister, bottle, alu...
-
Product Automated NIR Spectroscopy
The SPECTRA NIV is a high speed inspection platform for the non-destructive acquisition and analysis of NIR spectra of lyophilized products. NIR spectroscopy allows the determination of multiple parameters simultaneously such as moisture concentration, defects of the lyophilizate and the identification of ...
-
Product Analytical Services
Pharmaffiliates is providing all the Analytical Services (i.e. Method Development, Method Validation & Transfer, Stability Studies, Contract Analysis, etc)
-
Product Process Development
BioDuro-Sundia’s process development services support drug substance production, providing an efficient path to manufacture API or intermediates. Our extensive services support production of API for GLP toxicology studies, IND-enabling studies, clinical studies, and commercialization efforts. qq...
-
Product Res_Q
Res_Q helps IT and quality leaders eliminate validation debt, achieve peace of mind and untangle valuable resources. Res_Q's data-focused architecture and wide array of industry-supported applications prevent you from falling deeper into validation debt with every new software release.
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product Extractables & Leachables Testing
Nelson Labs Europe offers a comprehensive approach toward Extractables & Leachables testing for the pharmaceutical industry. Our approach combines technical and analytical expertise, polymer knowledge and understanding of regulatory requirements, all combined in a tailored approach for our customers.
-
Product Process Development services
Our early phase Process Development services focus on bridging the gap between lab scale and GMP manufacturing, ensuring robust scalability and outstanding productivity. In addition, based on our almost 20 years of experience, our late stage Process Development services design and execute process transfer ...
-
Product Analytical Testing and Formulation Development
Quality Chemical Laboratories is known for its capabilities in the development and validation of analytical methods for raw materials, drug substances, and drug products. With a strong focus on quality and precision, QCL employs cutting-edge technologies and a highly skilled team of scientists to offer com...
-
Product Cleaning Validation Analysis Support for Pharma Production
Our analytical scientists provide cleaning validation analysis from our GMP compliant laboratories. We ensure that fit-for-purpose analytical methods are specific for the substances being assayed and suitable to detect contaminants at the specified ARL for an appropriate level of cleanliness (sensitivity)....
-
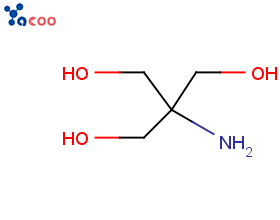
Product Cytidine (Cytidine)
Yamasa corporation offers a wide range of biochemicals products which includes cytidine (cytidine). Contact us for more information.
-
Product Validation Of Processes Service
Labanalysis offers a wide range of products which includes validation of processes service. It process validation activities in the field of sterilization filtration and performs analytical tests in order to optimize the choice of materials involved, technical support in validation processes. Contact us fo...
-
Product Freeze drying
The value proposition generated through two sites specialized in freeze drying is unique, from manufacturing process optimization to producing freeze dried characterized and documented vials, allowing our clients to diff erentiate on their own markets. We recently invested 7 million euros to increase capac...
-
Product Automation Engineering Solutions
We provide solutions and technologies that reduce human intervention and increase efficiency.
Automation is offered in DCS projects independent of the automation vendor. We offer professional services in implementing Automation process control and maintenance in accordance with industry standards ...
-
Product Training
Our Subject Matter Experts provide training that fulfill all regulatory requirements
Training is an integral part of GMPs. The operations involved in the manufacture of regulated products are highly technical in nature. Inadequate training of personnel can result in products failing to meet the ...
-
Product Toxicology
Our experts ensure clients' safety by protecting them from pharmaceutical hazards
At PharmEng Technology, we understand the importance of evaluating the toxicity and safety of biopharmaceuticals, therapeutic proteins, chemicals, active pharmaceutical ingredients, and components.
P...
-
Product Thermal Mapping
We protect the integrity and quality of your temperature sensitive pharmaceutical products to meet all regulatory requirements
PharmEng Technology has developed standards and methods to perform thermal process ratings according to your specifications. Our professionals are equipped with the righ...
Upcoming Events
-
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025 -
CPHI & PMEC China 2025
Shanghai New International Expo Center
24 Jun 2025 - 26 Jun 2025 -
CPHI South East Asia 2025
MITEC, Kuala Lumpur, Malaysia
16 Jul 2025 - 18 Jul 2025
Pharmaceutical Industry Webinars
Recommended Products And News
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance























































.png)



.png)







.png)

.png)



.jpg)





































































.jpg)
