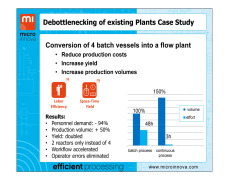
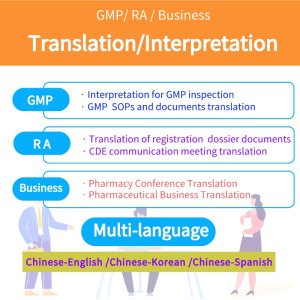
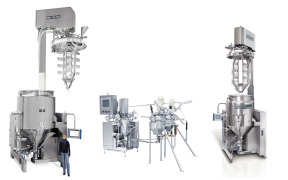
Thermal Mapping

Product Description
PharmEng Technology

-
ES
-
2022On CPHI since
-
2Certificates
-
250 - 499Employees
Company types
Primary activities
Categories
Specifications
PharmEng Technology

-
ES
-
2022On CPHI since
-
2Certificates
-
250 - 499Employees
Company types
Primary activities
More Products from PharmEng Technology (9)
-
Product Commissioning & Qualification
Our qualified consultants strive to exceed your project goals and objectives
Our flexibility and project experiences range from the qualification of an individual piece of equipment to an entire facility.
Our PharmEng professionals have extensive experience in the development, imple... -
Product Engineering
We help bring concept ideas to life
PharmEng engineers are experienced in building scalable and robust pharmaceutical production facilities and processes while employing state of the art techniques to meet and exceed operational and regulatory requirements.
Our experts have succes... -
Product Training
Our Subject Matter Experts provide training that fulfill all regulatory requirements
Training is an integral part of GMPs. The operations involved in the manufacture of regulated products are highly technical in nature. Inadequate training of personnel can result in products failing to meet the ... -
Product Project Management
Our experts incorporate the most modern and efficient Project Management techniques to ensure all project goals and objectives are met
PharmEng Technology has recognized that Project Management is essential throughout the Pharmaceutical, Biotechnology, and Medical Device industries.
... -
Product Quality Systems
We establish and manage quality systems specifically tailored to clients’ operational needs.
Our clients understand the importance of establishing a robust quality system; The effort in maintaining a strong quality system not only ensures that the products and services meet the customer expectat... -
Product Regulatory Affairs
We provide solutions to meet both local and global regulatory requirements in the most cost-effective manner.
PharmEng supports Pharmaceuticals, Biologics, Over-The-Counter (OTC), Animal Health, Radiopharmaceuticals, Clinical Trial Products, Medical Devices, Combination Products, Natur... -
Product Toxicology
Our experts ensure clients' safety by protecting them from pharmaceutical hazards
At PharmEng Technology, we understand the importance of evaluating the toxicity and safety of biopharmaceuticals, therapeutic proteins, chemicals, active pharmaceutical ingredients, and components.
P... -
Product Automation Engineering Solutions
We provide solutions and technologies that reduce human intervention and increase efficiency.
Automation is offered in DCS projects independent of the automation vendor. We offer professional services in implementing Automation process control and maintenance in accordance with industry standards ... -
Product Validation
Our team of experts will prepare necessary protocols incorporating all critical parameters
Process Validation:
PharmEng professionals possess experience in validating pharmaceutical and biopharmaceutical manufacturing processes. Our team of experts will prepare necessary protocols i...
PharmEng Technology resources (3)
-
News CPHI Frankfurt
PharmEng Technology was in #CPHI Frankfurt!!
The biggest international pharma industry event was taking place from the 1st to the 3rd of November.
We are waiting for you! Please feel free to contact. -
Brochure PharmEng Presentation
With this presentation you will be able to learn more about all the services that PharmEng Technology is capable of providing and you will understand why several of the most important companies worldwide trust us. -
Brochure PharmEng Brochure
With this brochure you will be able to have an overview about all the services that PharmEng Technology is capable of providing and you will understand why several of the most important companies worldwide trust us.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




















































-comp267516.png)