28
Oct
2025

BSP Pharmaceuticals S.p.a
Exhibitor at CPHI Frankfurt 2025 stand 5.0B50, Contract Manufacturing and Services
About Us
Categories

-
IT
-
2015On CPHI since
Event information
CPHI Frankfurt 2025
-
28 Oct 2025 - 30 Oct 2025
-
Messe, Frankfurt
-
Visit us at stand 5.0B50, Contract Manufacturing and Services
Products Featured at CPHI Frankfurt 2025
-
Product Clinical and Commercial supply services
BSP Pharmaceuticals S.p.A. offers a wide range of services which includes clinical and commercial supply services. It offers a high level of expertise to support the manufacturing of clinical products from phase I to phase III; prepare and complete PPQ manufacturing and support commercial product for world... -
Product Sterile suites capabilities
CLINICAL AND COMMERCIAL MANUFACTURING
A UNIQUE MANUFACTURING SYSTEM SPECIALLY DESIGNED FOR ANTICANCER DRUGS, WITH THE FLEXIBILITY TO MANAGE SMALL MOLECULES AND THE COMPLEXITY OF ADCs
-
Product Oral capabilities
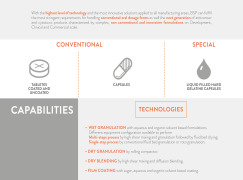
With the highest level of technology and the most innovative solutions applied to all manufacturing areas, BSP can fulfill
the most stringent requirements for handling conventional oral dosage forms as well the next generation of anticancer
and cytotoxic products characterized by complex, non con... -
Product ADCs Manufacturing services
BSP PHARMACEUTICALS IS SPECIALIZED TO SUPPORT THE MANUFACTURING OF ADC DRUG PRODUCTS FROM THE CONJUGATION TO THE FILL FINISH. Manufacturing lines, process flows as well as storage capabilities have been specially designed taking into consideration the critical proce... -
Product Quality control laboratories services
BSP Pharmaceuticals S.p.A. offers a wide range of services which includes quality control laboratories services. The facility houses chemical, biochemical and microbiological laboratories for the following typologies of analyses: analytical methods transfer/validation; product release testing and stability... -
Product Development services
BSP Pharmaceuticals S.p.A. offers a wide range of services which includes development services. It is conveniently situated in the production area of the plant. The specialists have the knowledge and training on the plant equipment and production requirements to reduce the risks of scale up failure. The se... -
Product Chemical laboratories quality control services
BSP Pharmaceuticals Srl offers a wide range of services which includes chemical laboratories quality control services. It includes analytical methods transfer, ich compliant method validation, in process, product release and stability testing, cleaning methods development and validation, raw materials test... -
Product Microbiological laboratories
BSP Pharmaceuticals S.p.A. offers a wide range of services which includes microbiological laboratories. Providing assays suitable for regulatory submission aligned with several regulatory agency requirements and equipped to carry out tests, such as: analytical methods validation, product release testing an... -
Product Biochemical laboratories
BSP Pharmaceuticals S.p.A. offers a wide range of services which includes biochemical laboratories. It has experience in establishing, validating and conducting routine bioassays according to cgmp standards. Fully validated assays suitable for regulatory submission are provided. Characterization work inclu...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance