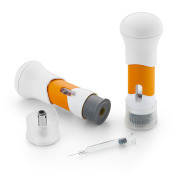
West Ready Pack™

Product Description
West Pharmaceutical Services, Inc.

-
US
-
2017On CPHI since
-
4Certificates
-
5000+Employees
Company types
Primary activities
Categories
West Pharmaceutical Services, Inc.

-
US
-
2017On CPHI since
-
4Certificates
-
5000+Employees
Company types
Primary activities
More Products from West Pharmaceutical Services, Inc. (14)
-
Product Selfdose™ patient-controlled injector
The SelfDose patient-controlled injector is an off-the-shelf delivery system that is ergonomically designed for optimal patient administration. -
Product Analytical services
West offers analytical services for specialized testing and analysis to evaluate the compatibility of drug products with their packaging or administration system for pharmaceutical and biopharma customers. -
Product Cartridge Systems
Delivery devices such as multi-use pens or pen injectors demand cartridge technology that’s predictable and safe, with tight dimensional tolerances, and that may be suitable for high-viscosity drugs and various storage conditions. See what West offers to help customers address these challenges. -
Product Flip-off® seals
Seals are a crucial part of a vial system. The selection of an appropriate seal is critical to ensuring container closure integrity, drug protection, user convenience and safety. -
Product Stoppers
West stoppers protect drugs from environmental impact and help maintain quality and safety. -
Product Daikyo® Crystal Zenith® 2.25mL Insert Needle Syringe System
The Daikyo® Crystal Zenith® (CZ) 2.25mL insert needle is a break-resistant, polymer syringe of choice to protect larger volume sensitive molecules during self-administration. For drug developers wishing to avoid glass, this syringe system protects delicate molecules as there is no silicone oil added for fu... -
Product Smartdose® On-Body Delivery System Platform (OBDS)
The SmartDose platform offers a wearable, subcutaneous injector with an integrated drug delivery system that incorporates human factors and usability testing to deliver a truly patient-centric approach to self-administration. -
Product NovaPure® Components
NovaPure® components have quality built in from the start. West has developed a comprehensive quality target product profile (QTPP) based on the needs of our customers and end users. -
Product Daikyo Crystal Zenith® Vials
With glass-like transparency, but superior break resistance and low risk of chemical interactions, Daikyo Crystal Zenith® vials can help avoid issues including delamination, and particulates. -
Product Daikyo Crystal Zenith® Syringe Systems
The Daikyo Crystal Zenith® Insert Needle and Luer Lock Syringe systems were designed to maintain purity, integrity and efficacy of premium biopharmaceutical therapies. -
Product Prefilled Syringes
An ideal choice for single- and multi-dose drugs, West components for prefillable systems help to ensure a more consistent injection rate to help patients receive accurate dosing with their treatment regimen. -
Product Contract Manufacturing Services
The West Contract Manufacturing team is focused on serving the needs of healthcare companies by providing a single-source solution from product conceptualization through manufacturing and final packaging.
West Pharmaceutical Services, Inc. resources (5)
-
News West Collaborates with Corning for Sustainable Drug Delivery
West and Corning Launch Viridian™ Vials that provide both performance and sustainability to the healthcare industry -
Video Examining Contamination Control Strategy in Primary Packaging as Part of EU GMP Annex 1
There are two major themes that involve primary packaging components in the revised EU GMP Annex 1 issued in August 2023. This recent revision includes: Implementing a holistic Contamination Control Strategy (CCS) Container Closure Integrity (CCI) because a properly seated stopper represents an adequate microbiological barrier. Pharmaceutical manufacturers must develop a CCS detailing the risks and mitigations within their operations, associated with contamination event opportunities with the emphasis being on personnel awareness, quality culture and controls, with focus on the principles involved in the protection of sterile product during the manufacturing, packaging and distribution processes.
This is why it is important to consider packaging decisions early in the development process. We will examine how primary packaging materials can impact your CCS and showcase West’s approach to mitigating risk on behalf of drug developers. -
Webinar Design of a New Elastomer Formulation for Lyophilization Applications
4040/40 elastomer formulation was designed with ‘future in mind’ to meet the ever-increasing performance & quality standards expected of primary drug packaging. The presentation will summarize the extensive Quality by Design (QbD) process that was undertaken to develop this new formulation as well as considerations taken for closure design dedicated for Lyophilization applications. Details of the extensive characterization that was performed as part of product development as well as data to demonstrates the overall performance of the new rubber formulation for lyophilization stoppers with respect to Extractables/Leachables, Particulates, Moisture, Container Closure Integrity (CCI), Particles and Manufacturability will be presented. -
Webinar Selection of a Primary Container Closure System Capable of Maintaining Closure Integrity during Cold Storage at -80°C
Advanced therapies are rapidly becoming a reality, targeting unmet patient needs and treatments for diseases with limited therapeutic options. Some advanced therapies, such as gene therapy and mRNA, require cryopreservation due to their fragile nature at room temperature, putting unprecedented expectations on the packaging systems to maintain Container Closure Integrity (CCI) during storage at -80°C. The quality, patient safety, and high value of these therapeutics can be jeopardized if the chosen containment system doesn’t consistently maintain a sterile barrier from when the drug is packed through to administration to the patient. During cooling between room temperature and -80°C, common vial system components such as glass vials, elastomeric closures, and aluminum seals exhibit contraction and changes in physico-mechanical properties. These changes in material properties and component dimensions may affect the system’s ability to maintain closure integrity, resulting in the formation of transient leaks.
In this joint study conducted by West and Corning, we discuss results from four experiments that explore the influences of capping pressure, cooling rate, dimensional changes, and long-term storage at -80°C on the system’s ability to maintain closure integrity. Both Helium leak testing and laser-based gas headspace analysis were used to monitor the closure integrity. The key result of the study is a combination of vial system components and capping conditions that maintain container closure integrity when cooled to and stored at -80°C -
Webinar How Market Trends Drive Innovation and Enable the Pharmaceutical Pipeline
Our healthcare industry is evolving. The move from IV to SC injection is being driven by lifecycle management, biosimilar adoption, and the hospital to home trend. Molecules are getting more complex, and patients drive the preference for self-administration resulting in the need for more complex combination product development and quick scale up. We will present innovative solutions to these challenges to help speed up drug development and enable patient choice with: A 2.25mL polymer container system for auto-injectors which protects sensitive molecules A large volume cartridge plunger to enable primary containment within an on-body delivery system A small pack, high quality glass containment system to enable quick delivery in early-stage development
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance