Contract Manufacturing Services

Product Description
West Pharmaceutical Services, Inc.

-
US
-
2017On CPHI since
-
4Certificates
-
5000+Employees
Company types
Primary activities
Categories
Specifications
West Pharmaceutical Services, Inc.

-
US
-
2017On CPHI since
-
4Certificates
-
5000+Employees
Company types
Primary activities
More Products from West Pharmaceutical Services, Inc. (11)
-
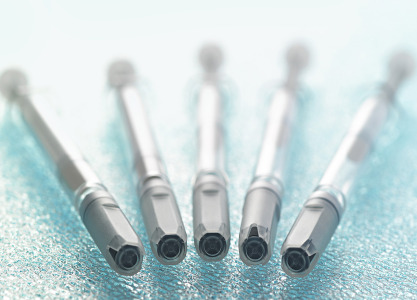
Product Daikyo Crystal Zenith(R) Vials
With glass-like transparency, but superior break resistance and low risk of chemical interactions, Daikyo Crystal Zenith(R) vials can help avoid issues including delamination, and particulates. -
Product Daikyo Crystal Zenith® Syringes
Daikyo Crystal Zenith® Insert Needle Syringe Systems - The syringe system was designed to maintain purity, integrity and efficacy of premium biopharmaceutical therapies. The system minimizes the potential contamination issues associated with glass and helps to reduce the risk of product interacti... -
Product West Ready Pack™ Containment Solution
Ready-to-Use Sterile Packaging
Our portfolio includes superior quality of ready-to-use stoppers, seals, and vials. The West Ready Pack containment solution demonstrates container closure integrity, giving you peace of mind that components work together, speeding up your time to market and reduci... -
Product SmartDose® On-Body Delivery System Platform (OBDS)
SmartDose® 3.5 On-Body Delivery System is a drug delivery system consisting of a battery-powered, wearable on-body injector with a separate, pre-fillable, polymer based cartridge. SmartDose® 3.5 OBDS incorporates human factors and usability testing to deliver a truly patient-centric approach to s... -
Product NovaPure® Plungers
NovaPure® plungers are made from West’s most modern, best-in-class elastomeric formulations with FluroTec™ barrier film to minimize compatibility issues with the drug product. From start to finish, NovaPure components have been designed and manufactured using Quality by Design principles to mitig... -
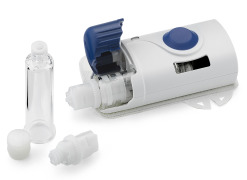
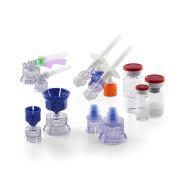
Product Vial Adapter™ Transfer Device
VIAL ADAPTERTM TRANSFER DEVICES - Options for siliconized spike and in-line particulate filters (available in 5µm, 15µm and 25µm pore sizes) VENTED VIAL ADAPTERTM TRANSFER DEVICES - One piercing spike with two lumens: One for transfer of drug/solution between the drug vial and a syringe and a secon... -
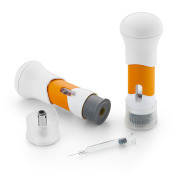
Product SelfDose™ Patient-Controlled Injector
TThe SelfDose patient-controlled injector is an off-the-shelf delivery system that is ergonomically designed for optimal patient administration. Extensive human factors studies have been performed with the SelfDose injector, confirming the intuitive design, supporting ease of use a... -
Product Analytical Lab Services
Analytical Services
The analytical services team has vast expertise and experience in extractables and leachables, particle analysis, container closure integrity, and performance and packaging/delivery systems among other methodologies. As a result of our understanding of materials and delivery ... -
Product Corning Vials
Corning® Viridian® Vials - provide the pharmaceutical industry with an optimized Type I borosilicate vial that reduces glass waste and CO₂e manufacturing emissions, while improving filling line performance. Viridian Vials are a drop-in solution made with 20% less glass material, enabling up to a ... -
Product Daikyo Crystal Zenith® (CZ) Ready-to-Use Nested Vials in Tub
Designed to meet the exterior dimensions of glass standard ISO-8362-1, CZ nested vials are a demonstrated containment solution for cell and gene therapies, radiopharmaceuticals, and sensitive molecules. CZ's attributes, such as maintaining container closure integrity down to cryogenic temperatures, superio... -
Product DAIKYO PLASCAP® RUV
Manufacturers seeking to rapidly change formats in a flexifiller can be challenged by a variety of issues, including availability of nested closures or aluminum particulate from seals. DAIKYO PLASCAP® RUV closures provide one-step assembly through an integrated stopper and plastic cap - helping to e...
West Pharmaceutical Services, Inc. resources (6)
-
News West Collaborates with Corning for Sustainable Drug Delivery
West and Corning Launch Viridian™ Vials that provide both performance and sustainability to the healthcare industry -
Video Examining Contamination Control Strategy in Primary Packaging as Part of EU GMP Annex 1
There are two major themes that involve primary packaging components in the revised EU GMP Annex 1 issued in August 2023. This recent revision includes: Implementing a holistic Contamination Control Strategy (CCS) Container Closure Integrity (CCI) because a properly seated stopper represents an adequate microbiological barrier. Pharmaceutical manufacturers must develop a CCS detailing the risks and mitigations within their operations, associated with contamination event opportunities with the emphasis being on personnel awareness, quality culture and controls, with focus on the principles involved in the protection of sterile product during the manufacturing, packaging and distribution processes.
This is why it is important to consider packaging decisions early in the development process. We will examine how primary packaging materials can impact your CCS and showcase West’s approach to mitigating risk on behalf of drug developers. -
Webinar Design of a New Elastomer Formulation for Lyophilization Applications
4040/40 elastomer formulation was designed with ‘future in mind’ to meet the ever-increasing performance & quality standards expected of primary drug packaging. The presentation will summarize the extensive Quality by Design (QbD) process that was undertaken to develop this new formulation as well as considerations taken for closure design dedicated for Lyophilization applications. Details of the extensive characterization that was performed as part of product development as well as data to demonstrates the overall performance of the new rubber formulation for lyophilization stoppers with respect to Extractables/Leachables, Particulates, Moisture, Container Closure Integrity (CCI), Particles and Manufacturability will be presented. -
Webinar Selection of a Primary Container Closure System Capable of Maintaining Closure Integrity during Cold Storage at -80°C
Advanced therapies are rapidly becoming a reality, targeting unmet patient needs and treatments for diseases with limited therapeutic options. Some advanced therapies, such as gene therapy and mRNA, require cryopreservation due to their fragile nature at room temperature, putting unprecedented expectations on the packaging systems to maintain Container Closure Integrity (CCI) during storage at -80°C. The quality, patient safety, and high value of these therapeutics can be jeopardized if the chosen containment system doesn’t consistently maintain a sterile barrier from when the drug is packed through to administration to the patient. During cooling between room temperature and -80°C, common vial system components such as glass vials, elastomeric closures, and aluminum seals exhibit contraction and changes in physico-mechanical properties. These changes in material properties and component dimensions may affect the system’s ability to maintain closure integrity, resulting in the formation of transient leaks.
In this joint study conducted by West and Corning, we discuss results from four experiments that explore the influences of capping pressure, cooling rate, dimensional changes, and long-term storage at -80°C on the system’s ability to maintain closure integrity. Both Helium leak testing and laser-based gas headspace analysis were used to monitor the closure integrity. The key result of the study is a combination of vial system components and capping conditions that maintain container closure integrity when cooled to and stored at -80°C -
Webinar How Market Trends Drive Innovation and Enable the Pharmaceutical Pipeline
Our healthcare industry is evolving. The move from IV to SC injection is being driven by lifecycle management, biosimilar adoption, and the hospital to home trend. Molecules are getting more complex, and patients drive the preference for self-administration resulting in the need for more complex combination product development and quick scale up. We will present innovative solutions to these challenges to help speed up drug development and enable patient choice with: A 2.25mL polymer container system for auto-injectors which protects sensitive molecules A large volume cartridge plunger to enable primary containment within an on-body delivery system A small pack, high quality glass containment system to enable quick delivery in early-stage development
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance























-file155014.png)



