Toxicology / Virology (Biopharmaceutical Products)
Toxicology / Virology (Biopharmaceutical Products) Companies (15)
Toxicology / Virology (Biopharmaceutical Products) News
-
News Women in Pharma: Advocating for trans healthcare in pharma
In our monthly series on women in the pharmaceutical industry, we interview leading experts in the pharmaceutical supply and value chain to discuss the importance of gender diversity in healthcare, the workplace, and beyond. -
News Sai Life Sciences to boost biology capabilities at its integrated R&D campus
The CRDMO will expand in vitro and in vivo biology services, DMPK and toxicology capabilities. -
News Development of rapid novel SARS-CoV-2 diagnostics started
Enzyme specialists from EUCODIS Bioscience and diagnostics experts from Biosynth-Carbosynth have teamed up to develop a novel diagnostic tool for the fast and reliable detection of an acute SARS-CoV-2 infection. Based on viral enzymatic activities and ... -
News GVK Bio continues to see growth tailwinds for 2020 thanks to a ‘crescendo of factors’
China-US trade challenges has led to de-risking by pharma companies.
Toxicology / Virology (Biopharmaceutical Products) Products (11)
-
Product Safety and Toxicity
- In vitro testing for skin corrosion (OCDE TG341);- In vitro testing for skin irritation (OCDE TG439 and ISO 10993-23:2021);
- In vitro testing for eye irritation – HET CAM (ICCVAM)In vitro testing for vaginal irritation (SOP Mattec Epivaginal);
- In vitro testing for Permeation studies of compounds...
-
Product Viral Vector Characterisation and Release Testing
Viral vector characterization and release testing services from Intertek's centre of Excellence for Biologics Characterisation, help you to establish and address critical quality attributes (CQAs) that impact product safety, purity, and potency. We deliver robust analytical assays to assess vector pro...
-
Product Cell bank and virus seed characterization
As a world leader in biopharmaceutical testing services, we offer you a comprehensive range of biologics safety testing , including virology, cell and molecular biology, as well as microbiology and electron microscopy. As a result, we can help you to ensure product safety and meet your regulatory requireme...
-
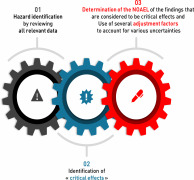
Product Calculation of PDE/OEL and Risk assessment reports of impurities
Performed by 6 DABT/ERT toxicologists.
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product Sterile/Toxic Isolator
Containment systems for sterile applications may be proposed in the form of closed isolators or Restricted Access Barrier Systems (RABS).
A sterile isolator allows to avoid the use of sterile cleanrooms, reducing the sterile volume around the process being executed.
Turbulent or unidirectional fl...
-
Product HPAPI Sterile Dispensing Isolator
Single chamber barrier isolator configured in order to grantthe dispensing procedure for API product achieving the followingtasks:• Assure a sterile environment inside the isolator. Assure operator protection, when the processedproducts are highly active.The barrier isolator operates under a positive ...
-
Product OEL/ADE/PDE Reports
Affygility Solutions has an extensive database of over 2,300 monographs.
Our OEL/ADE/PDE monographs prepared and endorsed by our in-house DABT/ERT certified toxicologists. This guarantees compliance with regulatory authorities such as EMA, PIC/S, ANVISA, and WHO.

-
Product Occupational Health Categorisation (OHC) Report
OHC reports are customised safety banding reports for compounds with limited data available. These compounds are usually early stage compounds with little or no human data available.
An OHC report will contain an occupational exposure band (OEB) and an estimated ADE value. The report&...
-
Product Safety Data Sheets (SDS)
Safety data sheets are used to determine the potential hazard of the materials being handled.
2 Weeks Turnaround Time
-
Product Permitted Daily Exposure monographs (" PDE ")
More than 1,000 API substances PDEs have already been developed in house.
New monographs are being developed on a constant basis.
Any PDE monograph can be developed upon request.
Our rates for PDE monographs are based on several factors such as status of development of the monogra...
Upcoming Events
-
Pharmapack Europe 2025
Paris Expo, Porte de Versailles - Hall 7.2 | Paris, France
22 Jan 2025 - 23 Jan 2025 -
CPHI Japan 2025
East Halls 4, 5 & 6, Tokyo Big Sight, Tokyo, Japan
09 Apr 2025 – 11 Apr 2025 -
CPHI Americas 2025
Pennsylvania Convention Center, Philadelphia
20 May 2025 - 22 May 2025
Pharmaceutical Industry Webinars
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance














.png)











.png)







.png)

.png)



.jpg)


