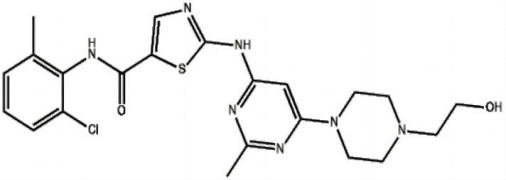
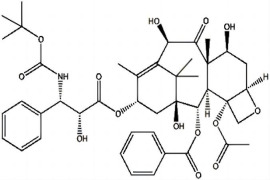
Shenzhen Haorui Industrial Dev. Co. Ltd
About Shenzhen Haorui Industrial Dev. Co. Ltd
Categories

-
CN
-
2015On CPHI since
-
100 - 249Employees
Company types
Meet us at
CPHI Frankfurt 2025
Messe, Frankfurt
28 Oct 2025 - 30 Oct 2025
Products from Shenzhen Haorui Industrial Dev. Co. Ltd (100)
-
Product Dasatinib anhydrous/monohydrate
We offer a wide range of API products which include Dasatinib monohydrate and Dasatinib anhydrous. It belongs to oncology category. Therapeutic category: molecular targeted. Regulatory status for Dasatinib monohydrate:USDMF No. 23763, USFDA Approved, Stability Studies Zone IVB, WC available ... -
Product Docetaxel anhydrous
We offer a wide range of API products which includes Docetaxel anhydrous. It belongs to oncology category. Therapeutic category: antineoplastic.
Other products:Amisulpride,Desvenlafaxine Succinate,Neostigmine methylsulfate
Teicoplanin,Daptomycin,Minocycline HCl,Vancomycin HCl, Levofloxacin... -
Product Irinotecan HCl
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of API products which includes Irinotecan HCl. It belongs to oncology category. Therapeutic category: antineoplastic.
Regulatory status of Irinotecan HCl:USDMF No. 23522, USFDA Approved,
CEP: R0-CEP 2020-025- Rev 00
Stability... -
Product Granisetron Base/Hydrochloride
We provide high quality Granisetron with DMF.Regulatory status for Granisetron base:
USDMF No. 22089, USFDA Approved
For Granisetron hydrochloride:
USDMF No. 19365, USFDA Approved
CEP: R1-CEP 2009-341-Rev 03, UK GMP
Stability Studies Zone IVB, WC available
If you want to know more informa... -
Product Flumazenil
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes Flumazenil. It belongs to API category. Therapeutic category: benzodiazepine.
Regulatory status of Flumazenil:
USDMF No. 17971, USFDA Approved
CEP: CEP 2008-121-Rev 02, UK GMP
Stability Studies ... -
Product Minocycline HCl
We offer Minocycline Hydrochloride of high quality with Eu GMP and DMF.Currently, there is a shortage of raw material in the world. However, our production capability is stable. -
Product Granisetron HCl
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of API products which includes Granisetron HCl. It belongs to antiemetic category. Contact us for more information. -
Product Epinastine HCl
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes Epinastine HCl. It belongs to API category. Therapeutic category: antihistaminic. Contact us for more information. -
Product Granisetron Base
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of API products which includes Granisetron Base. It belongs to antiemetic category. Contact us for more information. -
Product Amisulpride
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes Amisulpride. It belongs to API category. Therapeutic category: antipsychotic. Contact us for more information. -
Product Abacavir sulfate
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of products which includes Abacavir sulfate. It belongs to API category. Therapeutic category: antiviral. Contact us for more information. -
Product SN-38
Shenzhen Haorui Industrial Dev. Co., Ltd. offers a wide range of API products which includes SN-38. It belongs to oncology category. Therapeutic category: antineoplastic. Contact us for more information.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance